First Trimester Testing or Integrated Screening Offered Through Warde Medical Laboratory
Things are changing in the field of Prenatal Screening. The QUAD test has become the standard and most used second trimester prenatal screen in clinical laboratories today. However, testing is now available during the first trimester, and the integrated test, which requires testing both in the first and second trimester, recently has been shown to be the most specific and sensitive method (Wald; Wapner). This article reviews the types of testing available and those that are offered through Warde Medical Laboratory.
What is the difference between a Diagnostic and Screening test?
Before considering which type of test to do, it is important to recognize that at the present time, the available first and second trimester tests are not diagnostic tests, they are screening tests. Diagnostic tests, such as cytogenetics performed on an amniocentesis or chorionic villus sampling (CVS) are tests that provide definitive information about a pregnancy with false positives and false negatives being very uncommon. Unfortunately, these diagnostic tests require an invasive procedure that carries a risk for losing the fetus, and rarely may have complications for the mother. In order that only women at relatively high risks for having an affected pregnancy are offered such invasive diagnostic tests, screening tests are performed. These are tests that require history, accurate gestational age determination and a venepuncture for the mother. The advantage of these tests is that they do not carry the risk inherent with invasive tests. Careful pretest counseling is important so the patient understands the limitations of screening tests (false positives and false negatives) so that they will not be confused by the results.
History of Screening Tests for Down syndrome
Age as a Risk Factor
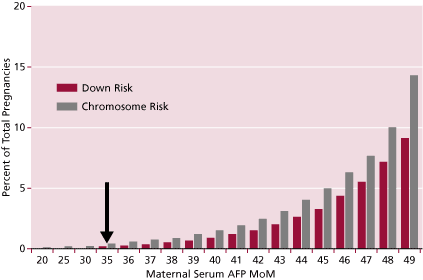
The original screening test was based upon the age of the mother. The risk for carrying a fetus with chromosomal abnormalities, especially Down syndrome, increases with the age of the mother. Down syndrome has an average incidence of 1 per 770 live births in all ethnic groups. But, as shown in Figure 1, its incidence increases with age. Since the 1970s it has been a common standard to offer amniocentesis or CVS to women who are 35 years of age or older at the time of delivery. Yet, there is a real risk of carrying a Down syndrome fetus at any age; that risk is much lower in younger women, and gradually increases continuously with age, accelerating with time. Therefore, since most pregnancies occur in younger women, even though their risk is lower than that of women 35 years of age or older, most Down syndrome babies are products of the younger women who are not being offered the more definitive invasive diagnostic tests.
Figure 1. Increase of Down and risk of all chromosomal abnormalities with age. Arrow shows risk at 35 years of age (typical cut-off used).
Second Trimester Screening Tests
Alpha fetoprotein (AFP)
In 1984, alpha fetoprotein (AFP) testing was offered to pregnant women because it was elevated when they were carrying a fetus with anencephaly or 80% of cases of open spina bifida (Figure 2). At that time, it was discovered that the serum levels of alpha fetoprotein tended to be lower in women with Down syndrome pregnancies (Merkatz, IR et al). This turned out to be a very weak screening test for Down syndrome, detecting about 20% of Down syndrome cases in women younger than 35 years. However, at that time it was the only practical way to identify women younger than 35 years who might be at a high enough risk that it was worth while to offer them the invasive diagnostic testing.
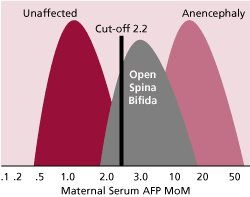
Figure 2. AFP multiples of the median (MoM) are shown for unaffected pregnancies and those with open spina bifida and those with anencephaly. The cut-off of 2.2 for Caucasian women detects about 80% of fetuses with open spina bifida.
Triple Test
Later, it was reported that the addition of human chorionic gonadotropin (hCG) and unconjugated estriol (uE3) to AFP in addition to considering the patient’s age could detect about 65% of Down syndrome cases (Haddow et al. 1992, 1998). The hCG is a strong predictor of Down syndrome. uE3, though only a relatively weak indicator of Down syndrome pregnancies turned out to be a useful marker for detecting Trisomy 18 (Edward’s syndrome), Smith-Lemli-Optiz syndrome (SLOS), and deficiencies of steroid sulfatase. One problem with this test was that all three analytes varied in concentration during the second trimester weeks that they were being tested (weeks 15-22.) (ACOG Practice Bulletin). Therefore, if there was an error in calculating the gestational age, such as due to an irregular menstrual period making calculation of the last menstrual period difficult, the patient may have been overestimated or underestimated for her risk due to this fact. Because of this issue, it has been noted that accurate gestational age from ultrasound reporting gives more true positives and fewer false positives. For instance, Benn reported that the Triple test detects 66% of cases of Down syndrome (when ultrasound was used to determine gestational age, but only 57% when LMP was used) with a false positive rate of 5% (5.8% when LMP was used to determine gestational age) (Benn, PA)
QUAD Test
The addition of dimeric inhibin A (DIA), producing the QUAD test, helped in this regard (Wald NJ). In addition to being a strong additional marker for Down syndrome, DIA changes only minimally in concentration during this period. Its addition to the calculation of Down syndrome risk ameliorated somewhat the effect of misdating the pregnancy resulting in a significant reduction in false positives (Table 1). With the QUAD test, Benn found that 73% (only 67% if LMP was used to calculate gestational age) of cases of Down syndrome were detected in women younger than 35, with a 4.6% false positive rate (5.5% if LMP was used).
Table 1. Decrease in false positives by use of the QUAD test. Down False + vs. Detection Foundation of Blood Research ASCP Teleconference 2001
| Markers* | False + | Detect Rate | Pos Amnio |
| Triple | 6.6% | 70% | 1:69 |
| Quad | 5.0% | 75% | 1:48 |
| 1.6 % x 17,000 cases screened = 272 fewer false + by Quad |
| * | Performed at Warde Medical Laboratory, Ann Arbor, MI |
For the past decade, second trimester testing for Down syndrome, Trisomy 18, and other abnormalities such as Smith-Lemli-Opitz syndrome (SLOS) has been the generally accepted standard. Last year, we performed just over 17,000 prenatal screens at Warde Medical Laboratory. By using the QUAD test we calculate that the false positives decreased by about 272 cases (Table 1). Because of the improved detection with decreased false positives, Warde Medical Laboratory recommends the QUAD test when second trimester testing is requested.
First Trimester Screening Tests
In the past several years, studies have been performed examining several methods to screen for cases of Down syndrome during the first trimester. Availability of an effective first trimester screen would allow women to choose CVS as a diagnostic test. In 1995, Wald et al were the first to report that two serum markers measured during the first trimester would detect 60-65% of cases of Down syndrome (at a 5% false positive rate) by using a combination of maternal age and measurement of pregnancy-associated plasma protein A (PAPP-A) and the free ![]() subunit of hCG (Wald et al, 1995; reviewed in Canick and Kellner, 1999). Although these numbers were similar to those of the Triple test, these percentages do not equate to the same numbers detected during the second trimester because there is a spontaneous loss of some Down syndrome pregnancies between the first and second trimesters. In addition, the second trimester quad marker test is superior to first trimester serum screening. Therefore, these early studies using only the two biochemical markers were not considered to be equivalent of the second trimester test, and because of this, few women availed themselves of first trimester serum screening.
subunit of hCG (Wald et al, 1995; reviewed in Canick and Kellner, 1999). Although these numbers were similar to those of the Triple test, these percentages do not equate to the same numbers detected during the second trimester because there is a spontaneous loss of some Down syndrome pregnancies between the first and second trimesters. In addition, the second trimester quad marker test is superior to first trimester serum screening. Therefore, these early studies using only the two biochemical markers were not considered to be equivalent of the second trimester test, and because of this, few women availed themselves of first trimester serum screening.
When performing first trimester testing, use of ultrasound gestational dating is strongly recommended because PAPP-A is exceptionally sensitive to gestational dating error. PAPP-A levels increase in the late first trimester by about 50% per week, a much bigger slope than seen with the other markers.
First Trimester Testing with Ultrasound NT Measurement
One of the key parameters for estimating the risk of carrying a fetus with Down syndrome during the first trimester is the ultrasound measurement of nuchal translucency (NT) thickness. Nuchal translucency is the subcutaneous, fluid-filled space between the cervical vertebrae and the skin. As originally used in screening, when the NT measurement was >95th percentile for a specific gestational age (determined by ultrasound dating), there is an increased risk for chromosomal abnormalities (especially Down syndrome). When the NT measurement by a trained ultrasonographer was combined with maternal age, 77% of first trimester cases of Down syndrome were detected with a 5% false positive rate (Snijders, RJM, et al). Since NT thickness is known to increase normally with increasing gestation, we now use a normalized NT value, usually the MoM, in assessing risk, as we do with each of the serum markers.
In 2003, Wapner et al reported a multicenter study where the combination of an accurate NT measurement with PAPP-A and free ![]() detected 85% of Down’s syndrome cases with a false positive rate of 9.4%. At a false positive rate of 5%, this corresponded to a detection rate of 79% of cases of Down syndrome in the first trimester (Wapner, R et al). However, when screening for Down syndrome is done in the first trimester, a second serum sample will be needed during the second trimester in order to measure AFP and estimate the risk for open spina bifida.
detected 85% of Down’s syndrome cases with a false positive rate of 9.4%. At a false positive rate of 5%, this corresponded to a detection rate of 79% of cases of Down syndrome in the first trimester (Wapner, R et al). However, when screening for Down syndrome is done in the first trimester, a second serum sample will be needed during the second trimester in order to measure AFP and estimate the risk for open spina bifida.
The key for these studies, however, is the ultrasonographic measurement. Even small differences in measurement can have significant effects on the risk determination. Sonographers who perform this assessment require training and ongoing quality monitoring in this measurement. The standards for this have been set by the Fetal Medicine Foundation of London (Snijders again). In addition, various professional groups within the United States, including SMFM and ACOG, are actively developing training and quality monitoring programs in NT scanning. (See “Documentation for NT measurement” for mechanism to be accredited for NT measurement).
Wald recently expressed concern about the use of the First trimester test to screen for Trisomy 21 and 18 because it gives an inferior performance to the Integrated test (see below) (Wald, NJ).
The Integrated Test (Combined First and Second Trimester testing)
In 1999, a new prenatal screen was described which would detect about 94% of cases of Down syndrome with a 5% false positive rate. At a 1% false positive rate, the new test was reported to detect 85% of cases, the same detection rate as the first trimester test but with only one-fifth the number of invasive diagnostic tests required. However, this screening test involved combining first trimester ultrasound NT information with testing performed on two samples of the mother’s serum. One obtained during the first trimester, from weeks 10 through 13, and a second sample obtained during the second trimester. Last year and earlier this year, two large clinical trials reported their results comparing first trimester, second trimester, and integrated tests. Both trials showed that the first trimester combined serum and ultrasound test and the second trimester quad test were similar in performance (between 80 and 85% detection rates for a 5% false positive rate), while the integrated test performance was considerably better (90-93% detection rate for a 5% false positive rate or 80-85% detection rate for a 1% false positive rate).
One negative feature of the integrated test is that because it requires assays during both the first and second trimester, there is a delay of over a month in most cases between the first sample and when the results are reported to the patient. This may increase the anxiety level in the patients. However, good acceptance by both the obstetricians and their patients has been seen due to the improved detection and lower false positive rates.
Another concern is that since the results of the first trimester testing are not independently provided to the pregnant woman, those who would test positive for carrying a Down syndrome fetus cannot avail themselves of the accurate, but invasive chorionic villus sampling (CVS) procedure that is done only in the first trimester. The reason the information is not given at the earlier time is that it defeats the strength of the integrated test, i.e. the information from the second trimester test helps to eliminate false positives from the first trimester data. False positives result in invasive procedures such as CVS or amniocentesis which carry a small risk of loss of the fetus.
If the NT information is not available, the patient can still take advantage of the Integrated Serum Screen that uses the biochemical assays performed on the two samples: one obtained at 10-13 weeks and another at 15-22 weeks. The assays for PAPP-A, AFP, hCG, uE3 and DIA have been shown to detect 84% of cases with 5% false positives.
Documentation for NT measurement.
The laboratory of Dr. Jacob Canick, at Women and Infants Hospital, Brown Medical School, in Rhode Island, the central laboratory for the recent First and Second Trimester Evaluation of Risk (FASTER) Trial, does the credentialing of ultrasonographers for Warde Medical Laboratory. For this a sonographer or group of sonographers in a practice can demonstrate that they have been trained in NT measurement by submitting at least 50-70 NT and Crown Rump Length (CRL) measurements for quantitative analysis. The NT is measured in tenths of mm, and the matching CRL is measured in mm. Dr. Canick’s laboratory determines whether the submitted NT data increase appropriately with gestational age, and whether after conversion to multiples of the median (MOMs), the NT values are distributed as expected. If these monitoring criteria are met, then the ultrasound center has established its own center-specific or operator-specific medians and can begin using NT measurements with our screening program. If the data do not match our expectations, we arrange for images to be reviewed by an expert in NT sonography, comments are returned to the sonographers, and then more NT and CRL values need to be submitted. Once a sonography center has begun working with our screening program, Dr. Canick’s lab will provide periodic quality assurance checks (bimonthly or quarterly, depending on volume) to make sure that the assigned medians have not drifted.
Summary
Clearly the state of prenatal screening continues to evolve. In the past 20 years we have gone from the AFP test designed mainly to detect open spina bifida to a QUAD test as the standard for second trimester testing. The integrated test which combines NT measurements with analytes from both the first and second trimester is the most sensitive and specific method currently available. However, the need for blood samples taken at two times and for specially trained ultrasonographers may be a barrier to widespread use at the present time. Table 2 outlines the testing currently available through Warde Medical Laboratory.
Table 2. Testing Offered at Warde Medical Laboratory
| Test | When (weeks) | Down Syndrome Detection (false+) | Open Neural Tube Detection (false+) | Markers Used |
| QUAD * | 15-22 | 75-80 (7%) | 80% (2%) | AFP, hCG, uE3, DIA |
| First Trimester** | 11-13 | 83% (5%) | Not Detected^ | PAPP-A, hCG, NT # |
| Integrated** Serum | 1st sample 10-13 2nd sample 15-22 | 84% (5%) | 80% (2%) | PAPP-A, AFP, hCG, uE3, DIA |
| Integrated ** NT Screen | 1st sample 10-13 2nd sample 15-22 | 90% (2%) | 80% (2%) | PAPP-A, AFP, hCG, uE3, DIA, NT # |
| * | Performed at Warde Medical Laboratory, Ann Arbor, MI |
| ** | Performed at Division of Prenatal and Special Testing, Brown Medical School, Women & Infants Hospital, Providence, RI |
| ^ | To detect open neural tube defects, a second sample is required between weeks 15-22 for the AFP assay. |
| # | NT – the nuchal fold translucency must be performed by an ultrasonographer who has been certified in this test. (Please see details in Documentation for NT Measurement). |
Further Reading
- ACOG practice bulletin no. 27, Prenatal diagnosis of fetal chromosome abnormalities. Washington DC; American College of Obstetricians and Gynecologists, 2001.
- Benn,PA, Advances in prenatal screening for Down syndrome: I. general principles and second trimester testing. Clin Chim Acta, 2002, 323, 1-16.
- Canick JA, Kellner LH. First trimester screening for aneuploidy: serum biochemical markers. Sem Perinatol 1999;23(5):359-68.
- Cuckle H. Biochemical screening for Down syndrome. Eur J Obstet Gynecol Reprod Biol 2000;92:97-101.
- Debieve F, Bouckaert a, et al. Multiple screening for fetal Down's syndrome with the classical Triple Test, dimeric inhibin A and ultrasound. Gynecol Obstet Invest 2000;49:221-226.
- Haddow JE, Palomacki GE, et a. Prenatal screening for Down's syndrome with use of maternal serum markers. NEJM 1992;327:588-593.
- Haddow JE, Palomaki GE, et al. Second trimester screening for Down's syndrome using maternal serum dimeric inhibin A. J Med Screen 1998;5:115-119.
- Krantz, DA et al. First-trimester Down syndrome screening: free bet-human chorionic gonadotropin and pregnancy-associated plasma protein A. Am J Obstet Gynecol 1996;1174:612-6.
- Lambert-Messerlian GM, Canick JA, et al. Second trimester levels of maternal serum inhibin A, total inhibin, alpha inhibin precursor, and activin in Down's syndrome pregnancy. J Med Screen 1996;3:58-62.
- Merkatz, IR et al. An association between low maternal serum alpha-fetoprotein and fetal chromosome abnormalities. Am J Obstet Gynecol 1984;148:886-94.
- Snijders, RJM, et al. UK multicentre project on assessment of risk of trisomy 21 by maternal age and fetal nuchal-translucency thickness at 10-14 weeks of gestation. Lancet 1998;352:343-6.
- Wald, NJ. First-trimester screening for Down's syndrome. NEJM 2004;350:619-620.
- Wald NJ, et al. Antenatal screening for Down's syndrome with the quadruple test. Lancet 2003;361:835-6.
- Wald NJ, Kennard, A. Hackshaw, A. K. First trimester serum screening for Down's syndrome. Prenatal Diagn 1995;15(13)1227-40.
- Wald NJ, Rodeck C, Hackshaw AK, Walters J, Chitty L, Mackinson AM. First and second trimester antenatal screening for Down's syndrome: the results of the serum, urine and ultrasound screening study (SURUSS). J Med Screen 2003;10:56-104.
- Wapner, R et al. First-Trimester screening for trisomies 21 and 18. NEJM 2003;349:1405-1413.
- Wenstrom KD, Owen J, et al. Alpha-fetoprotein, free beta-human chorionic gonadotropin, and dimeric inhibin A produce the best results in a three-analyte, multiple-marker screening test for fetal Down syndrome. Am J Obstet Gynecol 1997;177:987-991.
- Wenstrom KD, Chu DC, et al. Maternal serum alpha-fetorotein and dimeric inhibin A detect aneuploidies other than Down syndrome. Am J Obstet Gynecol 1998;179:966-970.
- Yoshida K, Kuwabara Y, et al. Dimeric inhibin A as a fourth marker for Down's syndrome maternal serum screening in native Japanese women. J Obstet Gynaecoll Res 2000;26:171-174.

