Serum Free Light Chain Assay in Diagnosis and Monitoring of Multiple Myeloma
Abstract
Detection of monoclonal free light chains (FLCs) is important for diagnosis and monitoring of monoclonal gammopathies. Although FLCs are especially important in light-chain diseases, such as light-chain myeloma, primary systemic (AL) amyloidosis, and light-chain deposition disease, FLC abnormalities occur in as many as 97% of plasma cell disorders, including 95% of intact immunoglobulin multiple myeloma cases and 82% of non-secretory multiple myeloma cases. Current methods for detection and quantitation of monoclonal proteins, such as protein electrophoresis and immunofixation electrophoresis, are insensitive for detection and quantitation of FLCs. An automated immunoassay that measures FLCs in serum and urine has recently been developed. Investigations into the clinical value of this assay indicate that many myeloma and AL amyloidosis patients would benefit from improved management through the use of the serum FLC assay. We describe here results from these studies, which suggest that inclusion of the serum FLC assay would improve the sensitivity of screening protocols for myeloma and AL amyloidosis; that the FLC assays, along with the size of the M-spike, can provide valuable information on prognosis of MGUS patients; and that including the FLC assays in monitoring protocols can reveal responses to treatment more rapidly than other assays.
Introduction
A distinguishing feature of the monoclonal gammopathies is the production of homogeneous populations of immunoglobulins by malignant clones of B-cells. Production of free light chains (FLCs) is an important feature of light chain diseases, such as light-chain myeloma (LCMM), primary systemic (AL) amyloidosis, and light-chain deposition disease (LCDD). However, in a retrospective study of 745 archived samples from patients with plasma cell disorders, 91% had abnormal FLC levels, and 97% had either elevated FLC levels or an abnormal ![]() ratio (see Table 1) [1-3]. Among those patients with intact immunoglobulin myeloma, 96% were found to have either elevated FLC levels or an abnormal
ratio (see Table 1) [1-3]. Among those patients with intact immunoglobulin myeloma, 96% were found to have either elevated FLC levels or an abnormal ![]() ratio.
ratio.
Table 1. Frequency of abnormal serum FLC concentrations and/or ratios
| Elevated concentrations of the involved free light chain | Concentration and/or ratio | ||||
| Disease | Total | Number | Percentage | Number | Percentage |
| IgG | 312 | 268 | 86% | 295 | 95% |
| IgA | 140 | 130 | 93% | 137 | 98% |
| IgD | 36 | 34 | 94% | 35 | 97% |
| IgE | 5 | 5 | 100% | 5 | 100% |
| LCMM | 224 | 224 | 100% | 224 | 100% |
| NSMM | 28 | 19 | 68% | 23 | 82% |
| Total | 745 | 680 | 91% | 719 | 97% |
These data suggest that the ![]() ratio may be of significant diagnostic value for plasma cell disorders. This was investigated by comparing sFLC results from 282 normal sera and 66 patients with multiple myeloma, primary (AL) amyloidosis, and light chain deposition disease [4]. The findings (shown in Table 2) indicate that the sFLC
ratio may be of significant diagnostic value for plasma cell disorders. This was investigated by comparing sFLC results from 282 normal sera and 66 patients with multiple myeloma, primary (AL) amyloidosis, and light chain deposition disease [4]. The findings (shown in Table 2) indicate that the sFLC ![]() ratio has considerable positive predictive value (PPV) and negative predictive value (NPV) for diagnosis of plasma cell disorders. These findings, and others, provide evidence that the sFLC assay can be valuable for diagnosis and monitoring of plasma cell disorders other than light chain myeloma, including intact immunoglobulin multiple myeloma (IIMM) [3] and non-secretory multiple myeloma (NSMM)2, and for prognosis in monoclonal gammopathy of undetermined significance (MGUS)5.
ratio has considerable positive predictive value (PPV) and negative predictive value (NPV) for diagnosis of plasma cell disorders. These findings, and others, provide evidence that the sFLC assay can be valuable for diagnosis and monitoring of plasma cell disorders other than light chain myeloma, including intact immunoglobulin multiple myeloma (IIMM) [3] and non-secretory multiple myeloma (NSMM)2, and for prognosis in monoclonal gammopathy of undetermined significance (MGUS)5.
Table 2. Diagnostic value of the diagnostic range for the ratio (0.26 – 1.65)
| Estimate (%) | 95% Confidence Interval (%) | |
| Sensitivity | 97 | 89 – 100 |
| Specificity | 100 | 98 – 100 |
| PPV | 100 | 91 -100 |
| NPV | 99 | 97 – 100 |
| Accuracy | 99 | 98 – 100 |
Quantitation of monoclonal immunoglobulins in serum, and sometimes urine, can provide information on disease course [6]. Although FLCs are commonly measured in urine, serum is to be preferred. FLCs are analyzed in urine by protein electrophoresis (UPE) and immunofixation electrophoresis (IFE) after concentration of the urine. Concentration of urine is time-consuming, labor-intensive, and can lead to protein loss from the specimen. Even more, the amount of FLCs in urine are strongly affected by renal function. With normal renal function, a significant amount of low molecular weight protein can be reabsorbed and metabolized in the proximal renal tubules. The quantity of low molecular weight protein that is reabsorbed in the proximal renal tubules has been reported to be between 1 and 30 g/24 hours [7-11]. This is to be compared with the normal production of FLCs, which is approximately 500 mg/24 hours [12]. Only 2 to 10 mg/L of FLCs are normally present in urine, and this is produced by mucosal cells in the distal renal tubules. For FLCs to make their way from blood into urine, the FLC level must be sufficiently high to cause overflow proteinuria, in which the level of FLCs overwhelms the ability for the proximal tubules to reabsorb filtered low-molecular weight proteins. The serum FLC level must be between 2 and 60 times the normal level for this to occur. Hence, urine FLC measurements may more accurately reflect renal tubule function, whereas serum FLC measurements may more accurately reflect status of plasma cell disorders.
FLC can be quantitated in serum protein electrophoresis (SPE) gels by scanning densitometry, but SPE is insensitive for FLC measurements. While the sensitivity of SPE is sufficient to quantify most intact immunoglobulins within their normal range, FLCs must be 20 to 200 times normal levels in order to be detected by SPE (see Tables 3 and 4). IFE is more sensitive than SPE, but is not quantitative, and as a result is not appropriate for monitoring. In addition, serum FLC levels must be approximately 10 to 15 times normal levels to be detected by IFE [13]. Hence, conventional electrophoresis methods are not sufficiently sensitive to measure FLCs at levels that are moderately elevated or in the normal range.
Table 3. Sensitivity of assays for serum free light chains [4, 13]
| SPE | 2,000 | 500 |
| IFE | 150 | 100 |
| sFLC assay | 0.5 | 0.6 |
Table 4. Reference intervals and diagnostic ranges
| 95 % reference interval (mg/L) | Diagnostic range | |
| 3.3 – 19.4 | ||
| 5.7 – 26.3 | ||
| 0.3 – 1.2 | 0.26 – 1.65 * | |
| IgG | 7,000 – 16,000 | |
| IgA | 700 – 4,000 | |
| IgM | 400 – 2,300 | |
| IgD | 0 – 80 |
* See text.
Recently, an alternative to SPE and IFE has been developed for quantitation of FLCs: an automated immunoassay that is three orders of magnitude more sensitive than either SPE or IFE [ 4, 13] (see Table 3). This level of sensitivity allows the serum FLC assays to be used to define both normal reference intervals and diagnostic ranges for free ![]() and free
and free ![]() light chains, and for the
light chains, and for the ![]() ratio [4] (see Table 4). In this study, a distinction was made between a reference range and a diagnostic range. The authors of this study stated: “the FLC K/L central 95% reference interval was 0.3-1.2. By definition, 5% of the general population will have a FLC K/L outside this 95% reference interval. If the FLC K/L is used as a diagnostic test for monoclonal FLCs and the FLC diseases, a 5% false positive rate is unacceptable. We therefore defined a FLC K/L diagnostic range that included all 282 reference sera tested in this study. The FLC K/L had a 100% range of 0.26-1.65.” [4]
ratio [4] (see Table 4). In this study, a distinction was made between a reference range and a diagnostic range. The authors of this study stated: “the FLC K/L central 95% reference interval was 0.3-1.2. By definition, 5% of the general population will have a FLC K/L outside this 95% reference interval. If the FLC K/L is used as a diagnostic test for monoclonal FLCs and the FLC diseases, a 5% false positive rate is unacceptable. We therefore defined a FLC K/L diagnostic range that included all 282 reference sera tested in this study. The FLC K/L had a 100% range of 0.26-1.65.” [4]
The ![]() ratio is of significant value, both for diagnosis and monitoring of patients with plasma cell disorders. An abnormal
ratio is of significant value, both for diagnosis and monitoring of patients with plasma cell disorders. An abnormal ![]() ratio suggests a clonal expansion of plasma cells. On the other hand, a normal
ratio suggests a clonal expansion of plasma cells. On the other hand, a normal ![]() ratio, in the presence of elevated levels of FLCs, suggests either renal impairment or a polyclonal expansion of plasma cells. In a study of serum FLC concentrations in non-secretory multiple myeloma patients, the
ratio, in the presence of elevated levels of FLCs, suggests either renal impairment or a polyclonal expansion of plasma cells. In a study of serum FLC concentrations in non-secretory multiple myeloma patients, the ![]() ratio was found to be a more sensitive diagnostic marker, due to suppression of the alternate light chain, than concentrations of the individual light chain [2]. In addition, monitoring the
ratio was found to be a more sensitive diagnostic marker, due to suppression of the alternate light chain, than concentrations of the individual light chain [2]. In addition, monitoring the ![]() ratio was found to be a useful method for distinguishing between the effects of renal damage and tumor growth (Figure 1).
ratio was found to be a useful method for distinguishing between the effects of renal damage and tumor growth (Figure 1).
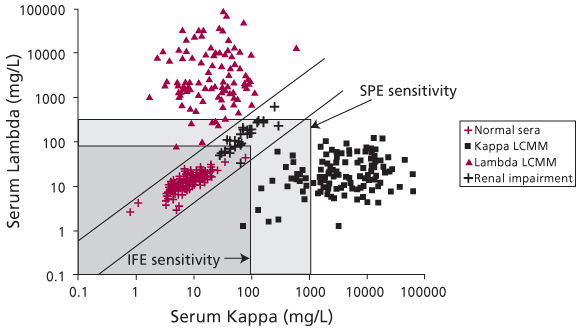
Figure 1. Serum FLC concentrations in normal individuals and patients with monoclonal gammopathies. This figure illustrates the utility of the ![]() ratio for diagnosis of monoclonal gammopathies. Quantitation of FLCs in normal sera shows a
ratio for diagnosis of monoclonal gammopathies. Quantitation of FLCs in normal sera shows a ![]() ratio between 0.26 and 1.65 (values between the diagonal lines). The
ratio between 0.26 and 1.65 (values between the diagonal lines). The ![]() ratio in patients with monoclonal gammopathies is either higher or lower than this range, such as shown here for Kappa Light Chain Multiple Myeloma (Kappa LCMM) or for Lambda LCMM. Note that an increase in FLC levels due to renal impairment or polyclonal gammopathy can be distinguished from a monoclonal gammopathy because patients with renal impairment or polyclonal gammopathy present with a normal
ratio in patients with monoclonal gammopathies is either higher or lower than this range, such as shown here for Kappa Light Chain Multiple Myeloma (Kappa LCMM) or for Lambda LCMM. Note that an increase in FLC levels due to renal impairment or polyclonal gammopathy can be distinguished from a monoclonal gammopathy because patients with renal impairment or polyclonal gammopathy present with a normal ![]() ratio (between the diagonal lines). The light gray box indicates the limit of sensitivity for SPE, while the dark gray box indicates the limit of sensitivity for IFE. The limits of sensitivity for SPE and IFE are as presented in Table 3 and published elsewhere [4, 13].
ratio (between the diagonal lines). The light gray box indicates the limit of sensitivity for SPE, while the dark gray box indicates the limit of sensitivity for IFE. The limits of sensitivity for SPE and IFE are as presented in Table 3 and published elsewhere [4, 13].
In addition to being highly sensitive, the serum FLC assay is also extremely specific. Serum concentrations of free light chains are several orders of magnitude lower than light chains bound to intact immunoglobulins. Hence, even a small amount of cross-reactivity would cause a significant increase in the reported levels of FLCs. In this light, it is of note that a study found the mean concentrations of FLCs in normal sera to be lower than had been previously reported [13]. Further, the serum FLC assay was able to detect suppression of the alternate (i.e., nonmalignant) FLC, supporting both good specificity and excellent sensitivity. When tested by immune precipitation and by hemagglutination, the serum FLC assay was found to have an insignificant amount of cross-reactivity with light chains bound to intact immunoglobulins and did not detect immunoglobulin heavy chains.
In this article, we will present evidence relating to the clinical value of the serum FLC assay for diagnosis and monitoring of patients with monoclonal gammopathies.
MGUS
Monoclonal gammopathy of undetermined significance (MGUS) represents approximately 56% of all monoclonal gammopathy patients [14]. MGUS is a premalignant condition that progresses to either myeloma or a related malignancy at a rate of about 1% per year [15], and presents a perplexing problem for physicians, inasmuch as it can be difficult to decide how frequently to monitor such patients, partly because of uncertainty about future progression.
Two recent studies suggest that the serum FLC assay can be used to stratify risk for MGUS patients, and then to help determine how often these patients should be tested. Rajkumar et al. [5] found that MGUS patients with an abnormal ![]() ratio are at significantly greater risk of progression than are MGUS patients with a normal
ratio are at significantly greater risk of progression than are MGUS patients with a normal ![]() ratio. Patients with a normal
ratio. Patients with a normal ![]() ratio and an M-spike of less than 1.5g/dL have an absolute risk of progression at 20 years of 7% (see Table 5) [16]. This is to be contrasted with patients who have both an abnormal
ratio and an M-spike of less than 1.5g/dL have an absolute risk of progression at 20 years of 7% (see Table 5) [16]. This is to be contrasted with patients who have both an abnormal ![]() ratio and an M-spike of at least 1.5g/dL: in these patients, the absolute risk of progression at 20 years is 46%. Clearly, monitoring should be conducted more frequently on patients who have both an abnormal
ratio and an M-spike of at least 1.5g/dL: in these patients, the absolute risk of progression at 20 years is 46%. Clearly, monitoring should be conducted more frequently on patients who have both an abnormal ![]() ratio and an M-spike of at least 1.5 g/dL.
ratio and an M-spike of at least 1.5 g/dL.
Table 5. MGUS risk stratification using the size of the serum M-spike and the FLC ratio
| Risk Group | Criteria | Number of Patients | Hazard Ratio | 20 yr Risk of Progression* |
| Low | M-spike < 1.5 g/dL and normal | 606 | 1 | 7% |
| Intermediate | Either M-spike or abnormal | 373 | 3.5 | 26% |
| High | Both M-spike and abnormal | 169 | 6.8 | 46% |
*Absolute risk of progression at 20 years
In both of these studies, the findings indicate that the presence of monoclonal FLC in serum, as detected by an abnormal ![]() ratio, is a major independent risk factor for progression of MGUS to either myeloma or a related malignancy. The new risk-stratification system described by Rajkumar et al. [16] identifies a low-risk group of patients that have a remarkably small life-time risk of progression, a finding of significant importance for the management of MGUS.
ratio, is a major independent risk factor for progression of MGUS to either myeloma or a related malignancy. The new risk-stratification system described by Rajkumar et al. [16] identifies a low-risk group of patients that have a remarkably small life-time risk of progression, a finding of significant importance for the management of MGUS.
Intact Immunoglobulin Multiple Myeloma (IIMM)
The serum FLC assay would present two areas of advantage over other laboratory methods for diagnosis and monitoring of IIMM patients. IIMM patients are monitored by following changes in total immunoglobulin levels, as measured by nephelometry, and in monoclonal immunoglobulins, as measured by densitometry of SPE gels. Both of these methods are insensitive at low levels. Hence, sensitive serum FLC measurements would be useful when monoclonal immunoglobulin production is low, especially if a significant proportion of IIMM patients produced excess levels of FLCs.
Retrospective analysis of archived serum from 493 patients with confirmed intact IIMM showed that 89% of patients had elevated levels of FLCs, and that 95% of patients had either elevated FLC levels or abnormal ![]() ratios [3]. In addition, in all cases in which the
ratios [3]. In addition, in all cases in which the ![]() ratio was abnormal, IFE results confirmed the light chain type of the tumors, providing a useful validation of the serum FLC results. Since the serum FLC results were not abnormal in all patients, SPE must be more sensitive for diagnosis of IIMM. However, the serum FLC assays have been shown to be more sensitive than SPE for diagnosis of LCMM and NSMM [1, 2]. Therefore, if a diagnosis of multiple myeloma is suspected, the optimal practice would be to screen sera by both SPE and FLC assays.
ratio was abnormal, IFE results confirmed the light chain type of the tumors, providing a useful validation of the serum FLC results. Since the serum FLC results were not abnormal in all patients, SPE must be more sensitive for diagnosis of IIMM. However, the serum FLC assays have been shown to be more sensitive than SPE for diagnosis of LCMM and NSMM [1, 2]. Therefore, if a diagnosis of multiple myeloma is suspected, the optimal practice would be to screen sera by both SPE and FLC assays.
A second advantage of the serum FLC assay is that treatment can be monitored much more effectively because of the short half-life of FLCs (see Table 6). The serum half-life of IgG is extended considerably, due to the actions of the ‘neonatal’ or ‘Brambell’ Fc receptor, which causes IgG to be recycled back into circulation rather than being catabolized [17]. As a result, the response to treatment in patients that are followed using IgG can appear to be much slower than patients that are followed using FLC. Further, following IIMM patients using the serum FLC assay can provide the benefits of the short half-life of FLCs, without the intrinsic problems and inaccuracies of urine assays [1,18]. In addition, the nonmalignant light chain can provide a convenient marker of bone marrow and renal function [3] (Figure 2).
Table 6. Half-life of immunoglobulins
| Type | Half-life | Percentage of IIM Patients |
| IgG | 20 -25 days | 59% |
| IgA | 6 days | 21% |
| IGD | 3 days | 1% |
| IgE | 2 days | 0.01% |
| FLC | 2 – 6 hours | 96% |
In one study, 17 IIMM patients were followed using serum FLC, intact immunoglobulin, and bone marrow plasma cell measurements [3]. Twelve of the 17 patients (8 of 12 IgG patients and 4 of 5 IgA patients) achieved a stable serological plateau. Serum FLC levels reached the normal range before the intact immunoglobulins in seven of these 12 patients (6 of 8 IgG patients and 1 of 4 IgA patients). In the other 5 patients that achieved a stable serological plateau (2 of 8 IgG patients and 3 of 4 IgA patients), the FLC and intact immunoglobulin levels arrived within the normal range at the same time. Thus, in 100% of patients that achieved a stable serological plateau during treatment, the FLC levels normalized either earlier than or at the same time as intact immunoglobulin measurements.
Twelve of the 17 patients were treated with high-dose melphalan and stem cell transplant. Eleven of these patients showed changes in the ![]() ratio towards normal and increases in the alternate light chain after treatment. Changes in the
ratio towards normal and increases in the alternate light chain after treatment. Changes in the ![]() ratio toward normal indicate that there was selective cytoreduction of the malignant cells following treatment. Bone marrow plasma cell counts were performed in six of the IgG patients after FLC levels were normal, and in all cases the plasma cells counts were < 10%. Thus, the serum FLC assay can provide valuable information regarding response to treatment, including being able to reveal the response to treatment earlier.
ratio toward normal indicate that there was selective cytoreduction of the malignant cells following treatment. Bone marrow plasma cell counts were performed in six of the IgG patients after FLC levels were normal, and in all cases the plasma cells counts were < 10%. Thus, the serum FLC assay can provide valuable information regarding response to treatment, including being able to reveal the response to treatment earlier.
Stabilization of FLC levels within the normal range may afford the opportunity to halt chemotherapy early. This can minimize damage to hematopoetic stem cells, which may be of benefit in patients that are destined for autologous stem cell transplants [19]. Early cessation of chemotherapy can also limit trauma to patients who are particularly frail and unsuitable for high-does therapy.
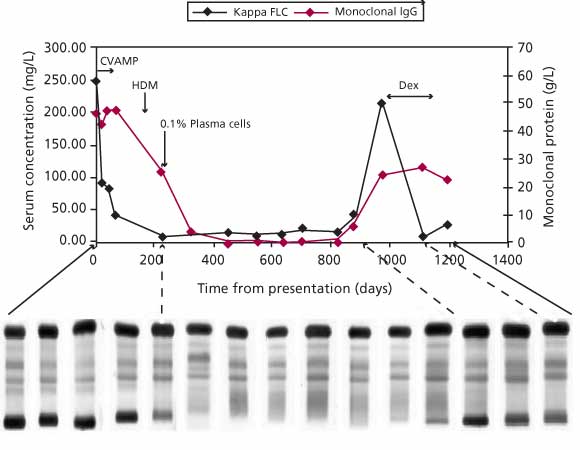
Figure 2. Following treatment in an IIMM patient with whole immunoglobulin, SPE, and serum FLC. Whole immunoglobulin was quantitated using nephelometry. Note that both sFLC level and IgG level fell following initiation of treatment, but that the sFLC level decreased sooner and reached plateau in advance of the IgG level. A bone marrow plasma cell count of 0.1% was made at the time when the sFLC level had fallen within in the normal range. At this time, there continued to be a considerable level of IgG present in serum. Relapse was detected by both serum FLC and whole immunoglobulin assays, but only the serum FLC assay detected the response to subsequent treatment with dexamethasone.
Light Chain Multiple Myeloma (LCMM)
LCMM accounts for approximately 15% of all multiple myeloma cases [20]. Two recent studies demonstrate the value of the serum FLC assay for diagnosis of LCMM. In one, presentation sera from 224 cases of LCMM, confirmed by bone marrow plasma cell counts and osteolytic lesions, were analyzed using the serum FLC assay [1]. The serum FLC assay correctly identified all 224 patients as having highly abnormal concentrations of FLCs. In this study, 82 patients were also followed using the serum FLC assay during treatment. During chemotherapy, 99% of patients (81 of 82) demonstrated a reduction in serum FLCs, while 95% (78 of 82) showed a reduction in urine FLCs. Only 11% of patients (9 of 82) demonstrated complete remission, defined as a normalization of serum concentrations of FLCs. In contrast, 32% (26 of 82) showed normalization of urine concentrations of FLCs. Monitoring patients using urine FLCs can give a mistaken impression of complete response, due to renal absorption of filtered FLCs [3]. Thus, the serum FLC assay is a sensitive marker for both diagnosis and monitoring of LCMM patients.
The goal of the second study was to determine if the serum FLC assay could replace measurement of FLCs in 24-hr urine for LCMM patients [18]. Out of a registry of 29,500 patients that is maintained at the Mayo Clinic, 28 LCMM patients were selected for analysis; previous 24-hr urine samples showed that 9 of these patients were positive for ![]() FLC and 19 patients positive for
FLC and 19 patients positive for ![]() FLC. All had a monoclonal FLC detectable by IFE in both serum and urine. However, only 3 of 9
FLC. All had a monoclonal FLC detectable by IFE in both serum and urine. However, only 3 of 9 ![]() patients and only 13 of 19
patients and only 13 of 19 ![]() patients demonstrated an M-spike in serum. Results of serum FLC assay analysis of these sera showed abnormal
patients demonstrated an M-spike in serum. Results of serum FLC assay analysis of these sera showed abnormal ![]() ratios for 100% of these patients. In addition, 8 of 9
ratios for 100% of these patients. In addition, 8 of 9 ![]() patients had above normal
patients had above normal ![]() concentrations and 19 of 19 lambda patients had above normal
concentrations and 19 of 19 lambda patients had above normal ![]() concentrations. Changes in urinary monoclonal protein were found to correlate strongly with changes in serum FLC concentrations when a random effects model was used, a finding that was later replicated by Alyanakian et al [21]. The authors of both studies concluded that the serum FLC assay is a logical and feasible alternative to measurement of monoclonal proteins in 24-hr urine samples.
concentrations. Changes in urinary monoclonal protein were found to correlate strongly with changes in serum FLC concentrations when a random effects model was used, a finding that was later replicated by Alyanakian et al [21]. The authors of both studies concluded that the serum FLC assay is a logical and feasible alternative to measurement of monoclonal proteins in 24-hr urine samples.
Non-secretory Multiple Myeloma (NSMM)
Patients with NSMM account for 1% – 2% of all multiple myeloma patients [20]. NSMM is distinguished, by definition, by the absence of monoclonal proteins detectable by conventional electrophoresis methods. Even so, approximately 85% of NSMM patients show cytoplasmic monoclonal proteins when examined by sensitive immunohistochemical methods [22]. Sensitive laboratory techniques can detect monoclonal proteins in urine from some of these patients, but conventional electrophoresis methods are inadequate for this purpose. As a result, diagnosis and monitoring of NSMM patients typically depends on clinical assessment and bone marrow biopsies.
Drayson et al. [2] investigated the clinical utility of the serum FLC assay in diagnosis and monitoring of NSMM. Sera were analyzed from 28 patients in whom NSMM had been confirmed by plasma cell content together with clinical symptoms of multiple myeloma, including lytic bone lesions or fractures. However, none of the patients were considered to have significant levels of M-protein in either serum or urine, using routine laboratory tests, at the time of presentation. Three of these patients (11%) had minimal Bence Jones proteins in urine by IFE. By contrast, 82% of patients (23 of 28) had abnormal serum FLC results: 19 patients showed either elevated FLCs or an abnormal FLC ratio; and additional 4 patients showed evidence of suppression of one or both FLCs. Repeat analysis of these sera by IFE showed monoclonal FLC in 6 of 28 patients (21%), but the monoclonal bands were weak and diffuse. In 9 of 28 patients (32%), IFE continued to be negative, even though the serum FLC levels were higher than 200 mg/L. Two of these 9 samples, selected at random, were found to contain highly polymerized FLC, with molecular weights of 40 – 200 kDa, as opposed to 25 kDa for monomeric and 50 kDa for dimeric FLC. Diffuse or poorly visible IFE bands may be explained by variable polymerization of FLCs, and polymerization of FLCs has been proposed as a molecular basis for NSMM [23]. In addition, polymerization would cause minimal renal clearance, which could account for negative urine tests in NSMM.
The same study examined the serum FLC assay for monitoring NSMM patients. Six patients were studied during the course of their disease. Analysis of sera by the serum FLC assay showed elevated FLC concentrations at presentation, significantly reduced levels during plateau phase, and increased levels at relapse in al six patients. Thus, the serum FLC assay is of use in the management of many patients with NSMM, and may reduce the need for bone marrow biopsies for tumor assessment.
AL amyloidosis
Primary (AL) amyloidosis is the most common and severe form of systemic amyloidosis, and is difficult to monitor quantitatively using conventional electrophoresis methods [24, 25]. SPE fails to detect a monoclonal protein at the time of diagnosis in about half of AL amyloidosis patients [25], and the concentration of monoclonal protein is so low in many other patients that quantitation is either imprecise or unfeasible. Although urine IFE can detect approximately 80 – 90% of patients [26], the results are not quantitative and are dependent on renal function (see discussion above).
Two studies examined whether the serum FLC assay is more sensitive than serum and urine electrophoresis methods. Lachmann et al. studied 262 patients with confirmed AL amyloidosis27. They found that only 53% (140 of 262) were detectable by SPE. Of the 47% of patients (122 of 262) that were negative by SPE, only 67 were positive by serum IFE (26%; 67 of 262), and 55 (21%; 55 of 262) were negative by IFE also. However, 98% were positive by the serum FLC assay (257 of 262). In the other study, Abraham et al. studied 95 patients with AL amyloidosis [28]. Their findings indicate that 39% (37 of 95) of patients were positive by serum IFE and 81% were positive by urine IFE (77 of 95), but that 91% of patients were positive by the serum FLC assay (86 of 95). Thus, the serum FLC assay is more sensitive for detection and diagnosis of AL amyloidosis than are electrophoretic methods.
Abraham et al. [28] examined the role of the serum FLC assay in monitoring treatment of AL amyloidosis patients by following 34 patients before and after stem cell transplant. Prior to transplantation, only about half of the patients could be monitored by either serum or urine M-protein levels (19 of 34 by SPE; 17 of 34 by UPE), while all 34 could be monitored by the serum FLC levels. Following transplantation, the serum FLC levels decreased earlier than either the urine total protein or the serum M-protein in 38% of patients (13 of 34). In 24% of patients (8 of 34), the serum FLC level was the only marker available to assess hematologic response. In another 29% (10 of 34) of patients, the decline in serum FLC levels was comparable to and occurred at the same time as urine total protein levels. In 9% of patients (3 of 32), the urine total protein levels fell prior to the serum FLC levels. Hence, treatment could be followed using the serum FLC assay in 100% of patients (compared with 56% by SPE and 50% by UPE), and the serum FLC assay revealed responses earlier than or at the same time as urine total protein in 91% of cases.
New hematologic response criteria for AL amyloidosis were recently proposed [29]. These new criteria represent a consensus among 13 leading clinicians from 6 countries. The new definition of partial response includes a reduction in serum FLC levels of 50%, and the new definition of complete response includes a provision that the
![]() ratio reach the normal range. Although some contend that the
ratio reach the normal range. Although some contend that the ![]() ratio should be maintained within the normal range for some period of time in order to justify a complete response [30], there is agreement that normalization of the
ratio should be maintained within the normal range for some period of time in order to justify a complete response [30], there is agreement that normalization of the ![]() ratio is a valid criterion for complete response. These criteria stem, in part, from a study of AL amyloidosis patients that demonstrated that survival at 5 years was 88% in patients that achieved at least a 50% reduction in serum FLC levels during treatment, while survival was only 39% in patients at the same time point if the reduction in serum FLC levels was less than 50% [27] (Figure 3). In the same group of patients, there was no difference in survival beyond 6 months among patients treated with IDM, VAD/C-VAMP, and HDM. Suppression of serum FLC by greater than 75% or 90% was not associated with significantly better survival than a decrease of just more than 50%. Thus, treatment strategies in AL amyloidosis may be guided by their effect on reducing the serum FLC concentration.
ratio is a valid criterion for complete response. These criteria stem, in part, from a study of AL amyloidosis patients that demonstrated that survival at 5 years was 88% in patients that achieved at least a 50% reduction in serum FLC levels during treatment, while survival was only 39% in patients at the same time point if the reduction in serum FLC levels was less than 50% [27] (Figure 3). In the same group of patients, there was no difference in survival beyond 6 months among patients treated with IDM, VAD/C-VAMP, and HDM. Suppression of serum FLC by greater than 75% or 90% was not associated with significantly better survival than a decrease of just more than 50%. Thus, treatment strategies in AL amyloidosis may be guided by their effect on reducing the serum FLC concentration.
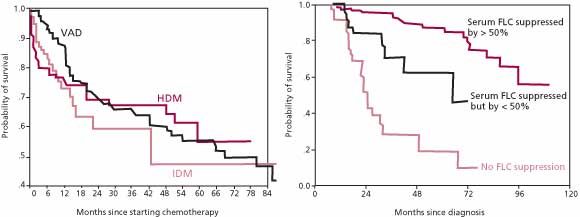
Figure 3. Kaplan-Meier estimate of survival in 137 patients with systemic AL amyloidosis. There were no significant differences between the degree of suppression of the clonal disease or the durability of the response among the patients who were treated with VAD, IDM, or HDM. In contrast, survival was significantly greater among patients who had a greater than 50% decrease in the concentration of the amyloidogenic class of FLCs.
In AL amyloidosis, the serum FLC assay is more sensitive for detection and diagnosis, allow a greater number of patients to be followed during treatment, can reveal responses earlier than other assays, and can be used to effectively guide treatment.
Summary
In summary, the serum FLC assay has been shown to be of clinical value in the diagnosis and monitoring of IIMM, LCMM, NSMM, and AL amyloidosis patients. The serum FLC assay can improve the sensitivity of screening protocols for myeloma and amyloidosis. Including the serum FLC assay in monitoring protocols can allow patients to be more effectively monitored and can reveal responses to treatment more rapidly. Further, new hematologic response criteria for AL amyloidosis were recently proposed; these new criteria include the serum FLC assays in the definitions of partial and complete responses. The serum FLC ratio has been found to be a major independent risk factor for MGUS, and the serum FLC ratio, together with the size of the M-spike, predicts the risk of progression for MGUS.
Conflict Statement: The authors of this article are associated with The Binding Site, the company that produces antisera used in the free light chain assay.
References
- Bradwell AR, Carr-Smith HD, Mead GP, Harvey TC, Drayson MT. Serum test for assessment of patients with Bence Jones myeloma. Lancet 2003; 361(9356): 489-91.
- Drayson M, Tang LX, Drew R, Mead GP, Carr-Smith H, Bradwell AR. Serum free light-chain measurements for identifying and monitoring patients with nonsecretory multiple myeloma. Blood 2001; 97(9): 2900-2.
- Mead GP, Carr-Smith HD, Drayson MT, Morgan GJ, Child JA, Bradwell AR. Serum free light chains for monitoring multiple myeloma. Br J Haematol 2004; 126(3): 348-54.
- Katzmann JA, Clark RJ, Abraham RS, Bryant S, Lymp JF, Bradwell AR, Kyle RA. Serum reference intervals and diagnostic ranges for free kappa and free lambda immunoglobulin light chains: Relative sensitivity for detection of monoclonal light chains. Clin Chem 2002; 48(9): 1437-44.
- Rajkumar SV, Kyle RA, Therneau TM, Clark RJ, Bradwell AR, Melton LJ, 3rd, Larson DR, Plevak MF, Katzmann JA. Presence of monoclonal free light chains in the serum predicts risk of progression in monoclonal gammopathy of undetermined significance. Br J Haematol 2004; 127(3): 308-10.
- Kyle RA. Sequence of testing for monoclonal gammopathies. Arch Pathol Lab Med 1999; 123(2): 114-8.
- Levinson SS, Keren DF. Free light chains of immunoglobulins: Clinical laboratory analysis. Clin Chem 1994; 40(10): 1869-78.
- McLaughlin P, Alexanian R. Myeloma protein kinetics following chemotherapy. Blood 1982; 60(4): 851-5.
- Abraham GN, Waterhouse C. Evidence for defective immunoglobulin metabolism in severe renal insufficiency. Am J Med Sci 1974; 268(4): 227-33.
- Solomon A, Waldmann TA, Fahey JL, McFarlane AS. Metabolism of Bence JSones proteins. J Clin Invest 1964; 43: 103-17.
- Wochner RD, Strober W, Waldmann TA. The role of the kidney in the catabolism of Bence Jones proteins and immunoglobulin fragments. J Exp Med 1967; 126(2): 207-21.
- Bradwell AR. Serum free light chain analysis, Second ed. Birmingham, UK: The Binding Site, Ltd., 2004.
- Bradwell AR, Carr-Smith HD, Mead GP, Tang LX, Showell PJ, Drayson MT, Drew R. Highly sensitive, automated immunoassay for immunoglobulin free light chains in serum and urine. Clin Chem 2001; 47(4): 673-80.
- Malpas JS, Bergsagel DE, Kyle RA. Myeloma: Biology and management, 1st. ed. Oxford; New York: Oxford University Press, 1995.
- Kyle RA, Therneau TM, Rajkumar SV, Offord JR, Larson DR, Plevak MF, Melton LJ, 3rd. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med 2002; 346(8): 564-9.
- Rajkumar SV, Kyle RA, Therneau TM, Melton LJ, Bradwell AR, Clark RJ, Larson DR, Plevak MF, Katzmann JA. Presence of an abnormal serum free light ratio is an independent risk factor for progression in monoclonal gammopathy of undetermined significance (mgus). Blood 2004; 104(11).
- Junghans RP, Anderson CL. The protection receptor for igg catabolism is the beta2-microglobulin-containing neonatal intestinal transport receptor. Proc Natl Acad Sci U S A 1996; 93(11): 5512-6.
- Abraham RS, Clark RJ, Bryant SC, Lymp JF, Larson T, Kyle RA, Katzmann JA. Correlation of serum immunoglobulin free light chain quantification with urinary Bence Jones protein in light chain myeloma. Clin Chem 2002; 48(4): 655-7.
- Tricot G, Jagannath S, Vesole D, Nelson J, Tindle S, Miller L, Cheson B, Crowley J, Barlogie B. Peripheral blood stem cell transplants for multiple myeloma: Identification of favorable variables for rapid engraftment in 225 patients. Blood 1995; 85(2): 588-96.
- Bradwell AR, Carr-Smith HD, Mead GP, Drayson MT. Serum free light chain immunoassays and their clinical application. Clinical and Applied Immunology Reviews 2002; 3(1-2): 17-33.
- Alyanakian MA, Abbas A, Delarue R, Arnulf B, Aucouturier P. Free immunoglobulin light-chain serum levels in the follow-up of patients with monoclonal gammopathies: Correlation with 24-hr urinary light-chain excretion. Am J Hematol 2004; 75(4): 246-8.
- Turesson I, Grubb A. Non-secretory or low-secretory myeloma with intracellular kappa chains. Report of six cases and review of the literature. Acta Med Scand 1978; 204(6): 445-51.
- Coriu D, Weaver K, Schell M, Eulitz M, Murphy CL, Weiss DT, Solomon A. A molecular basis for nonsecretory myeloma. Blood 2004; 104(3): 829-31.
- Buxbaum J. Aberrant immunoglobulin synthesis in light chain amyloidosis. Free light chain and light chain fragment production by human bone marrow cells in short-term tissue culture. J Clin Invest 1986; 78(3): 798-806.
- Kyle RA, Gertz MA. Primary systemic amyloidosis: Clinical and laboratory features in 474 cases. Semin Hematol 1995; 32(1): 45-59.
- Keren DF, Alexanian R, Goeken JA, Gorevic PD, Kyle RA, Tomar RH. Guidelines for clinical and laboratory evaluation patients with monoclonal gammopathies. Arch Pathol Lab Med 1999; 123(2): 106-7.
- Lachmann HJ, Gallimore R, Gillmore JD, Carr-Smith HD, Bradwell AR, Pepys MB, Hawkins PN. Outcome in systemic al amyloidosis in relation to changes in concentration of circulating free immunoglobulin light chains following chemotherapy. Br J Haematol 2003; 122(1): 78-84.
- Abraham RS, Katzmann JA, Clark RJ, Bradwell AR, Kyle RA, Gertz MA. Quantitative analysis of serum free light chains. A new marker for the diagnostic evaluation of primary systemic amyloidosis. Am J Clin Pathol 2003; 119(2): 274-8.
- Gertz MA, Comenzo R, Falk RH, Fermand J-P, Hazenberg BO, Hawkins PN, Merlini G, Moreau P, Ronco P, Sanchorawala V, Sezer O, Solomon A, Grateau G. Definition of organ involvement and treatment response in primary systemic amyloidosis (al): A consensus opinion from the 10th international symposium on amyloid and amyloidosis. Blood 2004; 104(11).
- Sanchorawala V, Wright DG, Magnani B, Skinner M, Seldin DC. Serum free light chain responses after high-dose melphalan and autologous stem cell transplantation for al (primary) amyloidosis. Blood 2004; 104(11).

