Nucleic Acid Testing for Chlamydia trachomatis and Neisseria gonorrhoeae
Background
Chlamydia trachomatis and Neisseria gonorrhoeae are the most frequently reported communicable diseases in the United States with 942,024 reported C. trachomatis infections and 327,541 reported N. gonorrhoeae cases in 2006 (1). The majority of men and women with C. trachomatis infection are asymptomatic and are not aware of their infection (2). Left untreated, C. trachomatis infections can lead to serious complications. Studies indicate that up to 40% of women with untreated C. trachomatis infections develop pelvic inflammatory disease (PID) (3, 4). Of these individuals, most have mild or nonspecific symptoms and do not seek medical treatment. The consequences of PID are severe, regardless of symptom severity. Twenty percent of women with PID will become infertile; 18% will experience debilitating, chronic pelvic pain; and 9% will have a life-threatening tubal pregnancy (5). Like chlamydial infections, uncomplicated N. gonorrhoeae infections are usually confined to the mucosa of the cervix, urethra, rectum, and throat. These infections are often asymptomatic in females and left untreated, N. gonorrhoeae infection can lead to PID, tubal infertility, ectopic pregnancy, and chronic pelvic pain (6).
C. trachomatis infection during pregnancy can lead to infant conjunctivitis, infant pneumonia, and maternal postpartum endometritis. N. gonorrhoeae can also be acquired at birth. Neonatal gonococcal infections can cause severe conjunctivitis which can produce blindness, sepsis, meningitis, endocarditis, and arthritis.
In males, urethritis is the most common symptom resulting from C. trachomatis and N. gonorrhoeae infections. Chlamydial complications (e.g., epididymitis) affect a minority of infected men and rarely result in long-term sequelae. Among men who engage in receptive anal intercourse, the rectum is a common site of C. trachomatis infection. While most rectal infections are asymptomatic, these infections can cause proctitis or proctocolitis. C. trachomatis can also cause conjunctivitis among adults and is a cause of sexually acquired reactive arthritis. Gonococcal infection in males usually manifests as symptomatic urethritis and with occasional epididymitis. In rare situations, local gonococcal infections will disseminate to cause an acute dermatitis tenosynovitis syndrome, which can be complicated by arthritis, meningitis, or endocarditis (6).
Assay Sensitivity
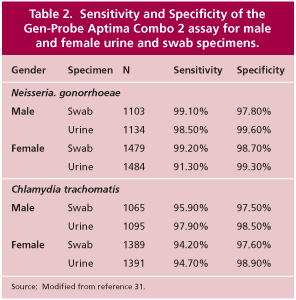
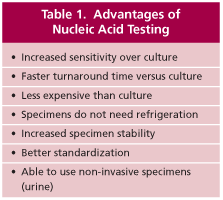 Culture testing for C. trachomatis and N. gonorrhoeae has been the reference standard against which all other tests have been compared. However, culture testing is being replaced by amplified nucleic acid testing because nucleic acid testing has a number of advantages over culture (Table 1). The primary drawback of culture, especially for C. trachomatis, is the lack of sensitivity. (7, 8, 9). Amplified nucleic acid tests are at least 20% to 30% more sensitive than culture (7, 8, 9) and nucleic acid tests can be used with noninvasively collected specimens such as first-catch urine specimens from men and women. The sensitivity of the Gen-Probe Aptima test used at Warde Medical Laboratory is shown in Table 2.
Culture testing for C. trachomatis and N. gonorrhoeae has been the reference standard against which all other tests have been compared. However, culture testing is being replaced by amplified nucleic acid testing because nucleic acid testing has a number of advantages over culture (Table 1). The primary drawback of culture, especially for C. trachomatis, is the lack of sensitivity. (7, 8, 9). Amplified nucleic acid tests are at least 20% to 30% more sensitive than culture (7, 8, 9) and nucleic acid tests can be used with noninvasively collected specimens such as first-catch urine specimens from men and women. The sensitivity of the Gen-Probe Aptima test used at Warde Medical Laboratory is shown in Table 2.
Role of Cryptic Plasmid. Most Chlamydiae isolates possess a 7.4 kbp cryptic plasmid designated pCT. This extrachromosomal genetic element was first isolated by Palmer and Falkow in 1986 (10). This cryptic plasmid was found in laboratory strains of all C. trachomatis serovars that cause human infection as well as in 200 separate clinical isolates. Genetic analysis of the plasmid revealed that the genetic sequences were very highly conserved (<1% variation) across all serovars (11). Because each Chlamydial organism contains 4 to 10 copies of pCT (10, 12, 13), nucleic acid tests that target cryptic plasmid sequences have increased sensitivity because there are more targets available. C. trachomatis DNA assays from three diagnostics manufacturers (Abbott, Becton Dickinson, and Roche) detect the presence of pCT sequences. However, several naturally occurring C. trachomatis strains lacking the plasmid have been isolated, including an L2 serovar cultured from a patient with proctocolitis (14), a genotype B variant cultured from a male urethral swab (15), and a serovar E cultured from a male urethral swab (16). Nine other plasmid-free specimens have been detected but these strains could not be isolated in culture (17). While plasmid-negative strains are thought to be rare (11) the possibility of Chlamydial infection caused by a plasmid-free strain DNA has implications when choosing a nucleic acid test for C. trachomatis and when treating symptomatic, Chlamydia-negative patients. The Gen-Probe assay used by Warde Medical Laboratory targets ribosomal RNA sequences and is not affected by the presence or absence of the cryptic plasmid.
Deletion Variants. In 2006, the Swedish Institute for Infectious Disease Control reported that a significant proportion of sexually transmitted Chlamydia trachomatis infections are caused by a genetic variant that has a 344 bp deletion in the cryptic plasmid. This deletion is in an area that is targeted by diagnostic tests marked by Abbott and Roche. Organisms containing this deletion can produce false negative results when tested with the Abbott and Roche molecular tests. Diagnostic tests from Becton Dickinson and Gen-Probe are able to detect this variant because they have different genetic targets. (18). It is unclear how long this deletion variant has been circulating in the Swedish population and the extent of the problem is still being assessed. However, the Swedish Institute for Infectious Disease Control reports that 39% of all Chlamydia cases during a one month period in unselected patients examined at primary health care/STI-/youth clinics were caused by the deletion variant (19). If this estimate is representative of the population at large, the failure to detect this genetic variant would have an adverse effect on the complication rates of genital Chlamydial infections. The high prevalence of this strain in some Swedish counties and the lack of detection and treatment have caused concern in other European countries (20, 21, 22).
Assay Specificity
The specificity of all nucleic acid tests is high for Chlamydia trachomatis (24). However, two commercially available, FDA cleared tests for Neisseria gonorrhoeae are known to cross-react with commensal Neisseria (9, 23, 24). This cross-reactivity has not been reported for the Gen-Probe assay used by Warde Medical Laboratory. Commensal Neisseria are recovered infrequently from the genitourinary tract but these frequencies can vary widely in different patient populations (25). Given the social impact of a false-positive N. gonorrhoeae result, Warde Medical Laboratory chose to use a test that did not cross-react with other Neisseria.
Test of Cure
Test-of-cure is not recommended as a routine procedure after completing treatment with doxycycline or azithromycin unless symptoms persist or reinfection is suspected (26). A test of cure may be considered 3 weeks after completion of treatment with erythromycin (e.g., in pregnant patients) (27). Tests of cure should not be performed <3 weeks after completion of antimicrobial therapy. Nucleic acid and direct fluorescent antibody tests performed <3 weeks after completion of antimicrobial therapy might be falsely positive because of the presence of nonviable organisms (9, 28, 29).
Pregnancy. Repeat testing (preferably by culture) 3 weeks after completion of therapy with an alternative treatment regimen is recommended for all pregnant women because these regimens may not be highly efficacious and the frequent side effects of erythromycin might discourage patient compliance with this regimen (27).
Test Selection. The validity of chlamydial culture testing following nucleic acid testing is questionable because 30% or more of specimens positive by nucleic acid amplification tests will be negative by culture (7). Nucleic acid testing is recommended as the test-of-cure following culture or nucleic acid screening.
Antibiotic Resistance. The CDC recommends that clinicians contact their local or state health department to arrange for antimicrobial susceptibility testing of isolates from patients apparently failing CDC-recommended therapy for C. trachomatis infection or CDC-recommended or FDA-approved therapy for N. gonorrhoeae infection (9).
Specimen Collection and Stability
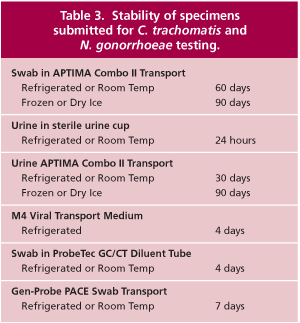
The Gen-Probe Aptima assays are approved for testing endocervical, vaginal, and urethral swabs and for male and female urine specimens (31). In addition to the Aptima transport tubes, Warde Medical Laboratory has validated C. trachomatis and N. gonorrhoeae testing on Gen-Probe PACE transports, M4 viral transport media, and BD ProbeTec CT/GC Diluent tubes. Testing of other specimen types and transport media has not been validated. The stability of the various specimen types are shown in Table 3. The Gen-Probe transport tubes are recommended by Warde Medical Laboratory because the nucleic acids are more stable in the Gen-Probe transports than in any other known system. With these transports, there is less likelihood of nucleic acid degradation during specimen shipping and handling.
Mucoid Specimens. The cervix of patients with Chlamydia or gonococcal infection is often coated with copious amounts of mucus. This mucus must be removed from the cervix using the absorbent white cleaning swab before sampling. The small blue sampling swab is then used to collect the specimen for testing. Only the blue sampling swab should be placed into the transport medium. This procedure is important because the mucus collected by the white cleaning swab can cause the transport medium to gel. This semisolid gel cannot be pipetted and the sample cannot be tested. Warde Medical Laboratory will reject any specimen that is submitted with the white cleaning swab in the transport medium.
Test Selection in Possible Sexual Assault or Abuse Cases
Specimen source. Endocervical specimens are appropriate for diagnosing C. trachomatis and N. gonorrhoeae infection of sexually active females. Because the immature vaginal epithelium of prepubescent females might be infected, specimens can be taken from the vagina of these patients.
Test Selection – C. trachomatis. Culture is the recommended method for detecting C. trachomatis in urogenital, pharyngeal, and rectal specimens. The chlamydia culture procedure at Warde Medical Laboratory utilizes standard isolation methods with fluorescent antibody staining of intracytoplasmic inclusions. This method is recommended by the Centers for Disease Control and Prevention (9). Direct fluorescent antibody tests for C. trachomatis are not recommended because they are not sufficiently sensitive and specific for testing victims or alleged assailants implicated in a sexual assault. The use of nucleic acid tests in suspected assault or abuse cases is still controversial. Some researchers suggest that amplified nucleic acid tests for C. trachomatis could be used as an alternative to cell culture if cell culture is unavailable and if another amplified nucleic acid test that targets a different sequence can be performed as an additional test if the initial nucleic acid test is positive (9). The nucleic acid testing algorithm at Warde Medical Laboratory meets this criteria but culture is still the preferred test method.
Test Selection – N. gonorrhoeae. Culture is the recommended method for detecting N. gonorrhoeae in urogenital, pharyngeal, or rectal swab specimens. Antigen tests and Gram-stained smears for N. gonorrhoeae are not sufficiently sensitive and specific for testing victims or alleged assailants implicated in sexual assaults (9).
Confirming Positive Tests
The CDC guidelines for screening tests to detect C. trachomatis and N. gonorrhoeae (9) state that an additional test might be indicated for a patients with a positive screening test result if a false-positive test would result in substantial adverse medical, social, or psychological impact for a patient. An additional test should also be performed if the positive predictive value is less than 90% (e.g., using a highly specific assay to test specimens from a low-prevalence population). Most of the patient populations served by Warde Medical Laboratory have low prevalence rates. Therefore, Warde Medical Laboratory confirms all positive screening tests by retesting the specimen using reagents that detect a different target sequence. By using two targets, this testing algorithm minimizes false positive results due to amplicon contamination and improves the reliability of the test result. The testing algorithm used at Warde Medical Laboratory is shown in Figure 1.
Specimens with a single test request (e.g., CHRNA for Chlamydia trachomatis) will be tested using the appropriate Aptima single test. Positive results will be confirmed using the Aptima Combo 2 assay that targets a different nucleic acid sequence. Because this test also detects N. gonorrhoeae, the laboratory will sometimes find a second pathogen in this specimen. Warde Medical Laboratory is required to report the presence of this pathogen to local health departments even if testing for this pathogen was not ordered by the physician. Warde Medical Laboratory will contact the requesting laboratory and will add a no-charge test for the additional pathogen so that its presence can be reported.
When the Chlamdyia trachomatis and Neisseria gonorrhoeae panel test (CHGCRNA) is ordered, the laboratory will use the Aptima Combo 2 assay as a screening test and the appropriate individual tests for confirmation.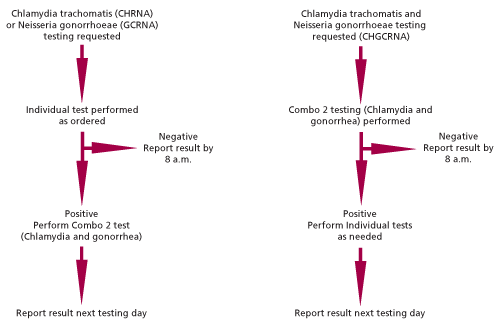
Figure 1:Testing algorithm for Chlamydia trachomatis and Neisseria gonorrhoeae testing. All positive results are re-tested using an assay that detects a second genetic target. The confirmatory test for specimens submitted with individual test requests will detect C. trachomatis and N. gonorrhoeae. Confirmatory testing for these specimens may therefore detect an additional pathogen that was not requested.
For More Information
Please contact Dr. Dan Wiedbrauk (Phone: 734-214-0300; E-mail: wiedbrad@trinity-health.org) for assistance in interpreting C. trachomatis and N. gonorrhoeae test results. Information regarding specimen collection, stability, sources, and testing status can be obtained from Dr. Wiedbrauk or the Molecular Biology Laboratory (Phone:734-214-0350).
Literature Cited
-
- Centers for Disease Control and Prevention. Provisional cases of selected notifiable diseases, United States, weeks ending December 23, 2006, and December 24, 2005. MMWR 2006;55:1385-1393.
-
- Stamm WE. Chlamydia trachomatis infections of the adult. In: Holmes KK, Sparling PF, Mardh P-A, Lemon SM, Stamm WE, Piot P, Wasserheit JN, eds. Sexually transmitted diseases. 3rd ed. New York, NY: McGraw-Hill, 1999:407–422.
-
- Rees E. Treatment of pelvic inflammatory disease. Am J Obstet Gynecol 1980;138:1042–1047.
-
- Stamm WE, Guinan ME, Johnson C, Starcher T, Holmes KK, McCormack WM. Effect of treatment regimens for Neisseria gonorrhoeae on simultaneous infection with Chlamydia trachomatis. N Engl J Med 1984;310:545–549.
-
- Westrom L, Joesoef R, Reynolds G, Hadgu A, Thompson SE. Pelvic inflammatory disease and fertility: a cohort study of 1,844 women with laparoscopically verified disease and 657 control women with normal laparoscopy results. Sex Transm Dis 1992;19:185–192.
-
- Hook EW III, Handsfield HH. Gonococcal infections in the adult. In: Holmes KK, Sparling PF, Mardh P-A, et al., eds. Sexually Transmitted Diseases. 3rd ed. New York, NY: McGraw-Hill, 1999:451–466.
-
- J. Schachter,J., Hook EW III, Martin DH, Willis D, Fine P, Fuller D, Jordan J, Janda WM, Chernesky M. Confirming positive results of nucleic acid amplification tests (NAATs) for Chlamydia trachomatis: all NAATs are not created equal. J. Clin. Microbiol. 2005;43(3):1372-1373.
-
- Schachter J. NAATs to diagnose Chlamydia trachomatis genital infection: a promise still unfulfilled. Expert Rev. Mol. Diagn.2001;1:137–144.
-
- Centers for Disease Control and Prevention. Screening tests to detect Chlamydia trachomatis and Neisseria gonorrhoeae infections — 2002. MMWR 2002;51(No. RR-15): 1-38.
-
- Palmer L, Falkow S. A common plasmid of Chlamydia trachomatis. Plasmid 1986;16:52-62.
-
- Anthony W. Solomon. AW , Peeling RW, Foster A, Mabey DCW. Diagnosis and assessment of trachoma. Clin. Microbiol. Reviews 2004;17(4):982-1011.
-
- Pickett MA, Everson JS, Clarke IN. Determination of chlamydial plasmid copy number using a fluorescent, 5'-exonuclease (TAQMAN) assay. In Saikku P, ed. Proceedings of the 4th meeting of the European Society for Chlamydia Research. Universitas Helsingiensis, Helsinki, Finland. 2000.
-
- Tam JE, Davis CH, Thresher RJ, Wyrick PB. Location of the origin of replication for the 7.5-kb Chlamydia trachomatis plasmid. Plasmid 1992;27:231-236.
-
- Peterson EM, Markoff BA, Schachter J, de la Maza LM. The 7.5-kb plasmid present in Chlamydia trachomatis is not essential for the growth of this microorganism. Plasmid 1990;23:144-148
-
- Farencena A, Comanducci M, Donati M, Ratti G, Cevenini R. Characterization of a new isolate of Chlamydia trachomatis which lacks the common plasmid and has properties of biovar trachoma. Infect. Immun. 1997;65:2965-2969.[Abstract]
-
- Stothard DR, Williams JA, Van Der Pol B, Jones RB. Identification of a Chlamydia trachomatis serovar E urogenital isolate which lacks the cryptic plasmid. Infect. Immun. 1998;66:6010-6013.
-
- An Q, Radcliffe G, Vassallo R, Buxton D, O'Brien WJ, Pelletier DA, Weisburg WG, Klinger JD, Olive DM ,1992. Infection with plasmid-free variant Chlamydia related to Chlamydia trachomatis identified by using multiple assays for nucleic acid detection. J. Clin. Microbiol. 1992;30(11):2814-2821.
-
- Ripa T, Nilsson P. A variant of Chlamydia trachomatis with deletion in cryptic plasmid: implications for use of PCR diagnostic tests. Euro Surveill 2006;11(11):E061109.2 Available from: (http://www.eurosurveillance.org/ew/2006/061109.asp#2)
-
- Söderblom T, Blaxhult A, Fredlund H, Herrmann B. Impact of a genetic variant of Chlamydia trachomatis on national detection rates in Sweden. Euro Surveill 2006;11(12):E061207.1. Available from: http://www.eurosurveillance.org/ew/2006/061207.asp#1
-
- Moghaddam A, Reinton N. Identification of the Swedish Chlamydia trachomatis variant among patients attending a STI clinic in Oslo, Norway. Euro Surveill 2007;12(3):E070301.3. Available from: http://www.eurosurveillance.org/ew/2007/070301.asp#3
-
- De Vries H, Catsburg A, van der Helm J, Beukelaar E, Morré S, Fennema J and Thiesbrumme H. No indication of Swedish Chlamydia trachomatis variant among STI clinic visitors in Amsterdam. Euro Surveill 2007;12(2). Available from: http://www.eurosurveillance.org/ew/2007/070208.asp#3
-
- Lynagh Y, Walsh A and Crowley B. Investigation to determine if newly-discovered variant of Chlamydia trachomatis is present in Ireland Euro Surveill 2007:12(2). Available from: http://www.eurosurveillance.org/ew/2007/070208.asp#4
-
- Roche Diagnostic Systems Inc. AMPLICOR™ Chlamydia trachomatis/Neisseria gonorrhoeae (CT/NG) (test package insert). Roche Diagnostic Systems, Inc., Branchburg, N.J. 2006.
-
- Becton, Dickinson and Company. BD ProbeTecTM ET Chlamydia trachomatis and Neisseria gonorrhoeae Amplified DNA Assays (test package insert). Becton, Dickinson and Company, Sparks, MD. 2006.
-
- Farrell DJ, Evaluation of AMPLICOR Neisseria gonorrhoeae PCR using cppB nested PCR and 16S rRNA PCR. J. Clin. Microbiol. 1999;27(2):386-390.
-
- Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines 2002. MMWR 2002;51(No. RR-6):32-42. 1-80.
-
- Bianchi A, Bogard M, Cessot G, Bohbot JM, Malkin JE, Alonso JM. Kinetics of Chlamydia trachomatis clearance in patients with azithromycin as assessed by first void urine testing by PCR and transcription-mediated amplification [Note]. Sex Transm Dis 1998;25:366–367.
-
- Gaydos CA, Crotchfelt KA, Howell MR, Kralian S, Hauptman P, Quinn TC. Molecular amplification assays to detect chlamydial infections in urine specimens from high school female students and to monitor the persistence of chlamydial DNA after therapy. J Infect Dis 1998;177:417–424.
-
- Morre SA, Sillekens PT, Jacobs MV, de Blok S, Ossewaarde JM, van Aarle P, van Gemen B, Walboomers JM, Meijer CJ, van den Brule AJ. Monitoring of Chlamydia trachomatis infections after antibiotic treatment using RNA detection by nucleic acid sequence based amplification. Mol Pathol 1998;51:149–154.
-
- Ferris DG, Lawler FH, Horner RD, Jernigan JC, Crout FV. Test of cure for genital Chlamydia trachomatis infection in women. J Fam Pract 1990;31:36–41.
- Gen-Probe Incorporated. Gen-Probe Aptima Combo 2 Assay (TIGRIS Application) (test package insert). Gen-Probe Incorporated, San Diego, CA. 2006

