Measuring Serum Free Light Chains (FLC) Improves Diagnosis and Prognosis for Patients with Monclonal Gammopathies

Figure 1: Title page of Dr. Henry Bence Jones’ original publication.
Bence Jones protein was the first biochemical tumor marker used to diagnose cancer. In 1848, Dr. Henry Bence Jones described the biochemical characterization of this important molecule (1). Today we know that the protein bearing his name is a monoclonal free light chain. Due to the recent development of an accurate method to measure free immunoglobulin light chains in serum, their evaluation has attained an indispensable role in the diagnosis and prognosis of a wide variety of pathologic conditions that have at their root the production of a monoclonal immunoglobulin.
For the past 20 years, immunofixation on concentrated urine has been the test of choice for detecting monoclonal free light chains in the urine (2). Unfortunately, immunofixation is not a quantitative procedure. Further, the best urine samples, an early morning void (before the patient has consumed a dozen cups of coffee) or a 24 hour urine collection (for measuring the monoclonal free light chains), are more difficult to obtain and deal with than serum.
The recent resurgence of interest in monoclonal free light chains relates to a relatively new technique that accurately measures free light chains in serum. Yes, serum, not urine. In 2001, Dr. Arthur Bradwell published a method that used highly specific antibodies purified to react only with free light chains, not to light chains bound to immunoglobulin heavy chains (3). The use of this method in clinical diagnosis has been reported in several hundred publications since Bradwell’s original report. At the Clinical Immunology Division Symposium of the American Association of Clinical Chemists meeting in San Diego this past July, Drs. Durie and Katzman emphasized the importance of this new test for detecting monoclonal gammopathies, helping to determine the prognosis for individual patients and in following the response to therapy.
Several practical recommendations are presented here for the use of the serum free light chain measurement.
Recommendation 1. In patients suspected of harboring a monoclonal gammopathy, serum free light chain measurements should be performed in addition to serum protein electrophoresis with immunofixation (or immunotyping—immunosubtraction).
Several studies, one performed at Warde Medical Laboratory, have indicated that addition of serum free light chain to routine serum protein electrophoresis improves the detection of clinically significant monoclonal gammopathies (4). Part of the improvement by being able to offer accurate assessment of serum free light chain values related to the difficulty , mentioned above, in obtaining urine samples to detect monoclonal free light chains. To determine whether using serum free light chains could eliminate the need for urine studies as part of the screen for multiple myeloma, Katzman et al. evaluated the results from 428 patient with a monoclonal gammopathy and monoclonal urinary protein at the initial diagnosis who had their serum free light chains measured within 30 days of diagnosis (5).
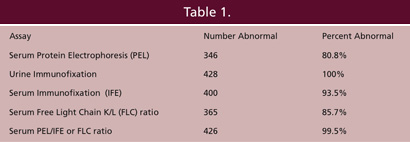
Data from Katzman et al. Mayo Clin Proc 81:1575-1578, 2006.
Presented at the American Association of Clinical Chemistry meeting, San Diego, July, 2007.
Since the inclusion criterion for the study was the presence of monoclonal free light chains in the urine, the study sought to determine how many of these cases would be detected if methods other than urine immunofixation were used to detect them. As shown in Table 1 from Dr. Katzmann’s study, serum protein electrophoresis alone missed 82 of these patients, almost 20% of the total. Serum immunofixation alone improved this result, missing 28 patients, about 6.5%. But, by adding the serum free light chain measurement (a positive result was considered if there was an abnormal serum free kappa/lambda ratio), to protein electrophoresis and immunofixation, only 2 patients (0.5%) were missed.
In a prospective study at Warde Medical Laboratory, Dr. Bakshi evaluated 1003 sera by both capillary zone serum protein electrophoresis and measurement of the serum free light chains (Table 2). He found 33 patients who had an abnormal serum free kappa/lambda ratio. Of those 33 cases, 16 of them had no monoclonal protein by serum protein electrophoresis. The light chain type that was increased agreed with the immunofixation in all cases where M-proteins were detected by immunofixation. Clinical follow-up was performed on these 16 cases. Nine of these patients were found to have B lymphocyte and/or plasma cell proliferative processes.
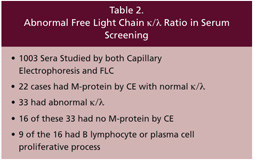 Data from Bakshi N. et al. Am J Clin Pathol Pathol 124:214-218, 2005.
Data from Bakshi N. et al. Am J Clin Pathol Pathol 124:214-218, 2005.
Both of these studies highlight the importance of ordering a serum free light chain test along with the protein electrophoresis and immunofixation to maximize the detection of monoclonal gammopathies.
Recommendation 2. In patients with MGUS (monoclonal gammopathy of undetermined significance), evaluation of the serum free light chains improves the prediction of progression.

Data from Rajkumar, et al. Brit J Haematol 106:812-17, 2005;
The vast majority of patients who have monoclonal gammopathies detected in serum end up being classified as MGUS. Kyle et al. define MGUS as the process in an individual who has a monoclonal gammopathy that is less than 3 G/dl, a bone marrow with less than 10% plasma cells and the absence of related organ or tissue damage (such as renal disease, anemia, hypercalcemia, etc) (6). When taken as a group, individuals with MGUS have about a 1% per year chance of progressing to multiple myeloma or other B cell lymphoproliferative disorder. However, recent data from Rajkumar et al, indicates that by combining the measurement of serum FLC with the amount of the monoclonal gammopathy and its isotype one can stratify the risk and provide individuals with a more precise estimate (7). Lower risks of progression are associated with an M-protein less than 1.5 G/dl, IgG isotype and a normal serum FLC κ/λ. When all 3 factors are in the low risk category, the risk of progression to disease and death in 20 years is 13-fold less than when these factors are in the high risk category (Table 3). Therefore, we recommend that upon the initial identification of a monoclonal gammopathy, the amount and isotype should be determined along with the serum FLC κ/λ.
Recommendation 3. In patients with multiple myeloma and a monoclonal free light chain, following the serum free light chain can detect responses to therapy faster than measuring the intact monoclonal protein.

Data from Mead et al. Br J Haematol 126:348, 2004
Currently, monoclonal proteins are followed by measuring the M-protein spike by densitometry (for agarose gels) or by electropherogram measurement (on capillary electrophoresis). Alternatively, some M-proteins migrate in the alpha2 or beta regions where they may be obscured by other proteins, necessitating the measurement of total immunoglobulin of the M-protein isotype by Nephelometry. However, intact immunoglobulins have much longer half lives than free light chains (Table 4). Mead et al. used FLC to follow the responses of 12 patients with multiple myeloma to chemotherapy (8). They found that in 7 of the 12, the FLC κ/λ returned to the normal range before the intact monoclonal immunoglobulin value declined. In following patients with high dose Melphalan and stem cell transplantation, 11 of the 12 patients showed change in the κ/λ towards normal with an increase in the non-involved free light chain value. Whereas serum FLC measurements are highly useful to detect and follow patients with monoclonal gammopathies, Dr. Kyle cautions that in addition to the serum FLC, we still need to perform a 24 hour urine on individuals who have had a monoclonal protein detected in the serum to see if and how much monoclonal protein is present in the urine. Further, therapy would depend on clinical evidence of recurrence, not just abnormal FLC levels.
Recommendation 4. Serum FLC measurements can detect the majority of patients with “Nonsecretory Myeloma.”
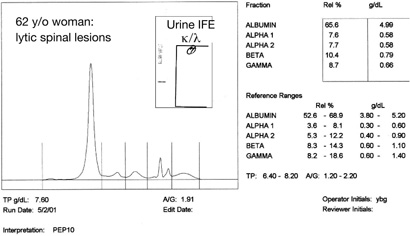
Figure 2: Capillary electophoresis and urine immunofixation (IFE) in a patient with “Nonsecretory Myeloma.”
The patient whose results are shown in Figure 2 had a normal serum protein electrophoresis, normal serum immunofixation and negative urine immunofixation for kappa and lambda. Yet, she presented with back pain, elevated serum calcium, lytic lesions on X-ray of her spine and had greater than 70% plasma cells in her bone marrow. Serum FLC κ/λ was 0.05 (normal range 0.26-1.65). She was diagnosed with nonsecretory myeloma. However, as reported by Drayson et al., she, like most individuals with nonsecretory myeloma did produce a monoclonal free light chain that could be measured with the serum FLC technique (9).
Nonsecretory myeloma may result from truncated immunoglobulin molecules or intracellular degradation. Further, a nonsecretory state may be acquired following chemotherapy. About 1-2% of cases of multiple myeloma are classified as nonsecretory. Yet, Drayson et al. found that 19 of the 28 cases they reviewed had either an abnormal serum FLC κ/λ or an elevation of one of the two light chain classes. Only 6 of the 19 were found to have tiny M-proteins detectable by immunofixation. While not a perfect solution to the problem of nonsecretory myeloma, FLC measurement allows an objective means to detect and potentially follow the majority of cases.
Recommendation 5. Use serum FLC measurements to help detect and follow therapy in patients with AL Amyloidosis.
AL Amyloidosis is the most common form of the disease. It results from deposition of monoclonal immunoglobulin light chain, either kappa or lambda, in tissues with disruption of function. It has long been known that by using immunofixation, most cases of AL Amyloidosis can be detected and followed. However, immunofixation is not a quantitative methodology and as many as 20% of cases have no detectable monoclonal protein by serum or urine immunofixation. Abraham et al. applied the serum FLC measurement to 95 individuals with AL Amyloidosis (10). Of 95 patients with documented AL Amyloidosis, 39% were positive by serum immunofixation, 81% were positive by urine immunofixation and 91% were positive by measurement of serum FLC. More impressive, however, was the use of FLC to monitor the response to therapy. There, while only 19 of the 34 patients demonstrated changes in the serum monoclonal spike, and only 17 had changes in their urine monoclonal spikes, all 34 had decreases in the abnormal FLC. In an interview with the Binding Site, Dr. Kyle cautioned that in individuals with AL Amyloidosis or in following therapy, while the abnormal FLC can be the earliest abnormality detected, currently therapy is not given until the patient has clinical manifestations of the disease.
Serum FLC measurements have become a valuable technique for improving initial detection of myeloma, AL Amyloid, nonsecretory myeloma as well as aiding in fine tuning the likelihood of progression in patients with MGUS.
References
-
- Jones, H. On a new substance occurring in the urine of a patient with mollities ossium. Phil Trans r Soc London 1848;138:55.
-
- Keren, DF, Protein Electrophoresis in Clinical Diagnosis. Arnold Publishing, London, 2003.
-
- Bradwell, AR et al. Highly sensitive, automated immunoassay for immunoglobulin free light chains in serum and urine. Clin Chem 2001;47:673.
-
- Bakshi N. et al. Am J Clin Pathol Pathol 124:214, 2005.
-
- Katzmann, JA, et al. Elimination of the need for urine studies in the screening algorithm for monoclonal gammopathies by using serum immunofixation and free light chain assays. Mayo Clin Proc 81:1575, 2006.
-
- Kyle, RA, et al. NEJM 356:2582, 2007.
-
- Rajkumar, et al. Brit J Jaematol 106:812-17,2005.
-
- Mead et al. Br J Haematol 126:348, 2004.
-
- Drayson et al. Blood 2001;97:2900.
- Abraham et al. Clin Chem 2002;48:655.

