Fourth Generation HIV Testing and New Diagnostic Algorithms for Detecting HIV Infection
 In April of this year, Warde Medical Laboratory switched to a fourth-generation screening platform for detection of infection by human immunodeficiency virus (HIV). The fourth generation platform — available for some time in Europe but only recently FDA approved in the US — differs from third generation platforms in the addition of detection of the HIV-1 p24 antigen in the initial ELISA screen. (1)
In April of this year, Warde Medical Laboratory switched to a fourth-generation screening platform for detection of infection by human immunodeficiency virus (HIV). The fourth generation platform — available for some time in Europe but only recently FDA approved in the US — differs from third generation platforms in the addition of detection of the HIV-1 p24 antigen in the initial ELISA screen. (1)
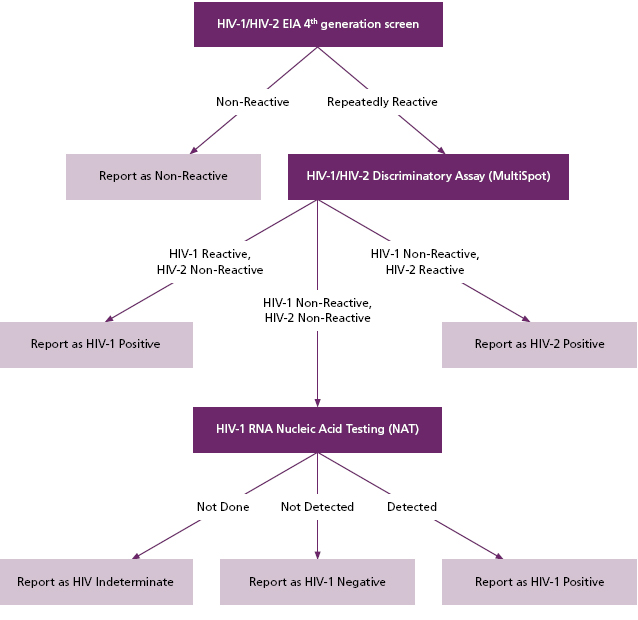
Fourth generation screening platforms also continue to detect antibodies directed against HIV-1, HIV-2, and HIV-O variant as was the case with the previous generation of HIV screening methods. In addition to updating the initial screen for HIV, Warde has also changed the positive screen follow up with testing that follows a diagnostic algorithm proposed by the U.S. Centers for Disease Control and Prevention (CDC) and the Association of Public Health Laboratories (APHL), and formally adopted by the Clinical Laboratory Standards Institute (CLSI) (Figure 1). (2)
According to this algorithm, the previous step of Western Blot confirmation is replaced by a rapid test for discrimination of HIV-1 from HIV-2 infection (“Multi-Spot”, BioRad), and, if necessary in the case of discordant results, nucleic acid testing (NAT) for HIV-1. Currently, two manufacturers (Abbott and BioRad) make FDA-approved fourth generation screening platforms. Warde is using the BioRad Evolis platform for this purpose. The rapid HIV-1/HIV-2
test as adopted by the CDC/APHL/CLSI algorithm is currently manufactured only by BioRad as the MultiSpot assay.
Figure 1. A new algorithm for HIV screening, as proposed by CDC/ APHL, and adopted by CLSI (Figure adapted from Styer et al, 2011)
There are several advantages to the new diagnostic algorithm.
First, the inclusion of p24 antigen detection in the initial screen allows for the earlier detection of new HIV infection by days or weeks compared to third-generation platforms. Studies have shown that the highest rate of HIV transmission occurs during a period of p24 antigenemia (generally detectable 2 to 3 weeks after initial infection) that precedes the development of detectable anti-HIV antibodies (which may take 3 to 12 weeks to develop to detectable levels). (3) It is hoped that the ability to detect HIV infection during this early window period may help to slow the rate of HIV transmission among the population.
Second, the new algorithm allows for the rapid distinction between antibodies directed against HIV-1 from antibodies directed against HIV-2. Currently, HIV-2 infection (still quite rare in North America) may cross-react with the HIV-1 Western blot, causing false-indeterminate or false-positive results ( Figure 1). (4)
Third, the MultiSpot assay will likely decrease the cost and turnaround time associated with determination of HIV status in infected individuals. While Western blot has high specificity and positive predictive value for HIV-1 infection, it is relatively insensitive compared to newer methods, and there is the potential for cross-reactivity with HIV-2. (4) Western blot may also have a relatively high rate of false-indeterminate results, especially in low-prevalence populations. Indeterminate results on HIV-1 Western blot (i.e. one or more bands present, but not fulfilling CDC criteria for a positive result) may indicate either a cross-reacting antibody in an HIV-negative patient, cross-reacting antibodies in an HIV-2 positive patient, or early HIV-1 infection. Therefore, indeterminate Western blot results currently are issued with a recommendation to re-test in 4 weeks to assess for the acquisition of additional bands that may indicate early HIV infection. While this has the potential to detect early HIV infection, it can also cause undue anxiety in HIV-negative patients who may harbor cross-reacting antibodies to HIV. Indeed, in a study performed by CDC of blood donors who screened positive for HIV and had indeterminate Western blot results due to antibodies against HIV GAG proteins, only 5 of 209 eventually seroconverted over a 2-year period, and those who did seroconvert did so on their first repeat sample. (5) At the time, the CDC indicated that individuals with persistent indeterminate Western blot results without acquisition of new bands should be considered HIV negative.
In the new algorithm, a repeatedly positive fourth generation screen will be tested via MultiSpot. If MultiSpot reveals antibodies to HIV-1, then the tested sample is considered positive for HIV-1. If the MultiSpot reveals antibodies to HIV-2, then the tested sample is considered positive for HIV-2. If the MultiSpot is negative in the setting of a positive fourth generation screen, then the algorithm proceeds to HIV nucleic acid testing (NAT). If NAT is positive for HIV-1, then the patient is considered positive for HIV-1. If NAT is negative for HIV-1, then the patient is considered negative for HIV-1. (6,7)
Referring clinicians will have to make individual determinations regarding any potential need for HIV-2 nucleic acid testing
in patients with positive initial 4th generation screens but non-reactive MultiSpot assays and negative HIV-1 NAT results. At present there is not an FDAapproved NAT assay for detection of primary HIV-2 infection, but non-FDA approved HIV-2 NAT is available as a sendout through Warde. Clients should keep in mind, however, that HIV-2 infection remains quite rare in individuals native to North America. 90% of reported HIV-2 cases in the US are from individuals who have immigrated from African nations, mainly in west Africa. Fewer than 170 cases of HIV-2 infection were confirmed by CDC between 1987 and 2009 (constituting only 0.01% of the more than 1.4 million cases of HIV infection during that time period), and only 3% of these cases (5 individuals) were born in the United States. (8)
Since NAT requires a sample that has not been previously tested (due to the sensitivity of NAT and concerns for contamination), and since NAT should be done within 72 hours of sample acquisition, Warde will report samples with positive 4th generation screens and negative MultiSpot assays as “HIV INDETERMINATE,” with a recommendation to pursue HIV-1 NAT on a repeat sample.
Results for specimens sent to Warde/MCL for primary screening for HIV will be reported in one of four ways, as outlined in Figures 2 through 5.
Since CDC/APHL/CLSI guidelines still allow for alternative diagnostic algorithms that include Western blot confirmation, Warde will continue to offer Western blot testing to those clients that wish to pursue this test after a positive HIV screen in their own laboratory. However, when a sample is sent to Warde for primary screening, positive screens will be tested via MultiSpot, rather than by Western blot.
| Figure 2 | |
| For patient samples non-reactive on initial fourth-generation EIA screen, the following result will be issued: | HIV NON-REACTIVE |
| Figure 3 | |
| For patient samples that are repeatedly reactive on the fourth-generation EIA screen and also reactive for HIV-1 on the MultiSpot Assay, the following result will be issued: | HIV-1 POSITIVE This sample was repeatedly reactive by a 4th generation HIV screening method, and also reactive for HIV-1 by the MultiSpot differentiation assay.According to an algorithm proposed by the CDC and adopted by the Clinical Laboratory Standards Institute (CLSI), a sample with this profile should be considered positive for HIV-1. If this is the first positive sample from this patient, we recommend repeat screening on a separately drawn sample. If further confirmation of a positive result is deemed necessary on clinical grounds, then HIV nucleic acid testing may be appropriate (refer to test code TMAHIV). |
| Figure 4 | |
| For patient samples that are repeatedly reactive on the fourth-generation EIA screen and also reactive for HIV-2 on the MultiSpot Assay, the following result will be issued: | HIV-2 POSITIVEThis sample was repeatedly reactive by a 4th generation HIV screening method, and also reactive for HIV-2 by the MultiSpot differentiation assay.According to an algorithm proposed by the CDC and adopted by the Clinical Laboratory Standards Institute (CLSI), a sample with this profile should be considered positive for HIV-2.If this is the first positive sample from this patient, we recommend repeat screening on a separately drawn sample. If further confirmation of a positive result is deemed necessary on clinical grounds, then HIV-2 nucleic acid testing may be appropriate. |
| Figure 5 | |
| For patient samples that are repeatedly reactive on the fourth-generation EIA screen but non-reactive for the MultiSpot Assay, the following result will be issued: | HIV INDETERMINATEHIV NUCLEIC ACID TESTING ON A REPEAT SAMPLE IS RECOMMENDED (TEST CODE TMAHIV)This sample was repeatedly reactive by a 4th generation HIV screening method, but was non-reactive for both HIV-1 and HIV-2 by the MultiSpot differentiation assay.According to an algorithm proposed by the CDC and adopted by the Clinical Laboratory Standards Institute (CLSI), a sample with this profile should be evaluated by HIV nucleic acid testing. Nucleic acid testing cannot be performed on the same specimen used for the initial screen. Please resubmit a new specimen for HIV-1 nucleic acid testing (refer to test code TMAHIV for proper sample requirement). |
Bibliography
- Titus, Karen. In new HIV guideline, 20+ years of change. CAP Today 26, no. 3 (2012): 1.
- Clinical Laboratory Standards Institute. Criteria for laboratory testing and diagnosis of human immunodeficiency virus infection; approved guidelines (CLSI document M53-A). Vols. 31, no. 13. Wayne, PA: Clinical Laboratory Standards Institute, 2012.
- Das, G, P Baglioni, and O Onyebuchi. Primary HIV infection. BMJ 341 (2010): c4583.
- Torian, Lucia V, Lisa A Forgione, Amado E Punsalang, Robert E Pirollo, and William R Oleszko. Comparison of Multispot EIA with Western blot for confirmatory serodiagnosis of HIV. Journal of Clinical Virology 52S (2011): s41-s44.
- Centers for Disease Control (CDC). Interpretation and use of the western blot assay for serodiagnosis of human immunodeficiency virus type 1 infections. MMWR 38(suppl 7) (1989): 1-7.
- Masciotra, Silvina, J Steven McDougal, J Feldman, Patrick Sprinkle, Laura Wesolowski, and S Michele Owen. Evaluation of an alternative HIV diagnostic algorithm using specimens from seroconversion panels and persons with established HIV infections. Journal of Clinical Virology 52S (2011): S17-S22.
- Styer, Linda M, and Timothy J, Parker, Monica M Sullivan. Evaluation of an alternative supplemental testing strategy for HIV diagnosis by. Journal of Clinical Virology 52S (2011): S35-S40.
- Centers for Disease Control and Prevention (CDC). HIV-2 infection surveillance — United States 1987-2009. MMWR 60, no. 29 (2011): 985-986.

