New Testing Options for Hepatitis C Virus
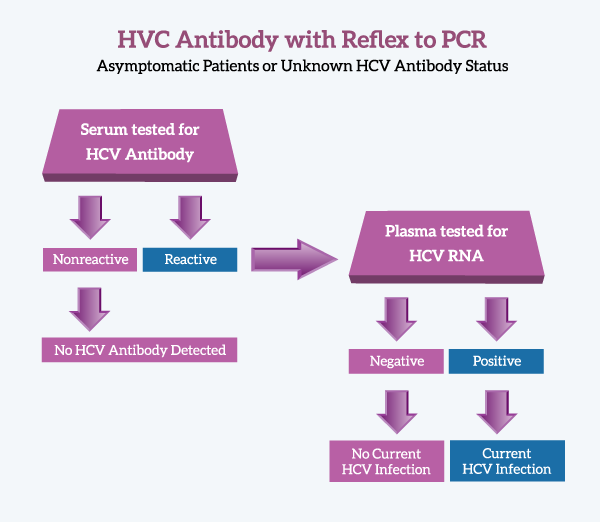
 Warde Medical Laboratory has a new testing option for diagnosing Hepatitis C Virus infections. The test, “Hepatitis C Virus Antibody with Reflex to PCR” includes HCV antibody testing with automatic referral to PCR for antibody-Reactive specimens. A final interpretation, based upon CDC guidelines, is provided for all specimens.
Warde Medical Laboratory has a new testing option for diagnosing Hepatitis C Virus infections. The test, “Hepatitis C Virus Antibody with Reflex to PCR” includes HCV antibody testing with automatic referral to PCR for antibody-Reactive specimens. A final interpretation, based upon CDC guidelines, is provided for all specimens.
This testing protocol requires submission of two separate sample tubes–one serum, the other plasma. The serum tube is tested for HCV antibody and if Reactive, the plasma tube is tested for HCV RNA by a quantitative PCR protocol.
Frequently Asked Questions
What are the advantages of this testing algorithm?
This testing protocol provides a “One and Done” specimen collection protocol for HCV virus testing that is relatively simple and cost-effective. Previous protocols required a second draw for HCV Antibody Reactive patients. Requiring a second draw is inconvenient for the patient and the temporal dissociation associated with collecting a new specimen may confuse the medical picture.
This testing protocol also provides an interpretative comment that reflects the CDC guidelines for HCV testing. These comments are not available on normal PCR tests.
When should this test be ordered?
The HCV Antibody with Reflex to PCR test can be used when screening asymptomatic patients and when testing patients/sources after an occupational exposure.
What test should be ordered for patients with acute hepatitis?
Patients with acute hepatitis should be tested with an HCV RNA PCR test because:
- HCV antibody results may be negative. HCV antibodies are not detectable for 8-12 weeks after infection.
- Symptomatic patients usually have detectable viremia.
- Viremia usually occurs soon after, or concomitant with, increases in ALT levels.
- In most patients, HCV RNA can be detected in blood within 1 to 2 weeks after infection
Why do we need two tests to diagnose Hepatitis C Virus?
The main reason for using a two-test algorithm is cost. HCV infection is relatively rare in the general population with 0.7 RNA-positive cases/100,000 population. PCR testing is 7 to 10 times more costly than antibody testing. Therefore, PCR screening is not cost effective, particularly in light of the disease incidence.
Unfortunately, antibody testing cannot provide all the necessary information for evaluating patients. This does not mean that HCV antibody tests are bad, it means that antibody tests cannot distinguish current active infections from cleared infections. Only active infections require medical follow-up.
Up to 25% of HCV-infected individuals clear the infection without antiviral intervention. These individuals will be antibody-positive but PCR-negative. This profile will persist for years after clearing the infection. Patients who have undergone successful antiviral treatment (sustained responders) will have a similar HCV profile. HCV RNA testing is required to distinguish current infections from cleared infections.
Why does Warde use a quantitative PCR test rather than a qualitative test?
Once again, the answer has to do with cost, but in a different way. There is no difference in cost for a quantitative (viral load) or a qualitative (Detected/Not Detected) HCV PCR test. The performance characteristics of these tests are exactly the same. In addition, the quantitative (viral load) test also includes a qualitative (Detected/Not Detected) answer. By using the quantitative test, we can provide more information at no additional cost.
How do I collect and process the specimens?
| DRAW TWO TUBES: | |
| SST or red-top tube for antibody testing | Lavender top tube for PCR testing |
| Allow blood to clotCentrifuge and separate serumTransfer serum (1.0 mL) to plastic, screw-capped vial.Refrigerate or freeze | Centrifuge and separate plasma within 6 hours of collection.Transfer plasma (3.0 mL) to plastic, screw-capped vial.Freeze plasma at -20oC |
What is the stability of the samples?
| Stability – Serum | Stability – Plasma |
| Room Temp: Not acceptable Refrigerated: 5 days Frozen (-20°C): 14 days | Room Temp: Not acceptable Refrigerated: 3 days Frozen (-20°C): 60 days |
What happens to the plasma tube if the HCV Antibody test is negative?
On receipt at Warde, the plasma tube is placed into frozen storage pending the antibody testing result. Once the HCV testing is complete, the plasma specimen may be used for add-on testing. The specimen is discarded after 2 weeks.
What happens if I don’t send a plasma tube?
For antibody-negative specimens, there are no significant consequences. If the specimen is Reactive in the antibody test, we will enter a QNS (Quantity Not Sufficient) result into the PCR fields and finalize the test. (The HCV antibody result is not visible to the client until all the fields are complete.) Under this scenario, the client will need to re-draw the patient and order the Hepatitis C Virus Supplemental PCR, Quantitative test (see below).
We perform HCV testing at our facility. How can I get the confirmatory testing done?
Order the Hepatitis C Virus Supplemental PCR, Quantitative test. This procedure assumes the specimen is HCV antibody-Reactive and provides CDC recommended test interpretations.
Can I use the normal HCV qualitative or quantitative tests as confirmatory tests following HCV antibody testing?
The Hepatitis C Virus (HCV) RNA, Qualitative and the Hepatitis C Virus (HCV) RNA, Quantitative assays can be ordered, but those tests do not provide the CDC-recommended result interpretations.
Why can’t Warde use one tube for the antibody and the PCR tests?
The answer has to do with contamination control and the relative sensitivity of the antibody and PCR tests. In modern chemistry and immunochemistry analyzers, all of the samples are open and the same probe is used to sample every specimen. While these analyzers have few contamination problems for analytes present in milligram concentrations, their anti-contamination protocols aren’t stringent enough when testing at the sub-nanogram level. PCR testing is >1000 times more sensitive than antibody testing and transferring a nanoliter of sample from one tube to another can change a negative result to positive. The use of dedicated specimens is important to prevent false-positive results.
Have more questions? Contact Warde Medical Laboratory at 734-214-0300.
Additional Information and Recommendations
- Moorman AC, Drobenuic J, Kamili S. Prevalence of false-positive hepatitis C antibody results, National Health and Nutrition Examination Study (NHANES) 2007–2012 Journal of Clinical Virology 89 (2017) 1–4
- Centers for Disease Control and Prevention. Recommendations for the Identification of Chronic Hepatitis C Virus Infection Among Persons Born During 1945–1965 (MMWR 2012;61(RR04);1-18).
- Centers for Disease Control and Prevention. Testing for HCV Infection: An Update of Guidance for Clinicians and Laboratorians. MMWR 2013; 62(18): 362-5.
- Centers for Disease Control and Prevention. Updated U.S. Public Health Service Guidelines for the Management of Occupational Exposures to HBV, HCV, and HIV and Recommendations for Postexposure Prophylaxis. MMWR 2001; 50 (RR11): 1-42.