Anticardiolipin Antibodies (ACA): Antigenic Targets and Pathophysiology
Antiphospholipid antibodies (APA) are recognized as the most common cause of acquired thrombophilia. APAs are detected by two different test systems. Lupus anticoagulants (LA) are identified by prolongation of the Activated Partial Thromboplastin Time (APTT) or an abnormal dilute Russell Viper Venom Time (dRVVT). ACAs are routinely identified by solid phase enzyme-linked immunosorbent assays (ELISA). The presence of positive results for LA or ACA together with clinical findings of arterial or venous thromboembolic events or recurrent fetal loss (RFL) identify patients with the antiphospholipid syndrome (APS). (Table 1) This review focuses on ACA testing and the clinical application of test results for patient management.
| Table 1 ANTIPHOSPHOLIPID SYNDROME (APS) | |
| CLINICAL | LABORATORY |
| Venous Thrombosis | IgG ACA (Med to High) |
| Arterial Thrombosis | IgM ACA (Med to High) |
| Recurrent Fetal Loss | Positive LA Testing |
| Thrombocytopenia | |
| In order to establish the diagnosis of APS, it is necessary to identify a well documented clinical complication(s) (VT, AT, RFL, thrombocytopenia) and the presence of persistently positive laboratory results. Retest the patient 8 – 12 weeks after the first documented positive ACA test.Wilson et al. Arthritis Rheum 42:1309-1311, 1999. |
Historical Perspective
![]() A.P. von Wassermann described a complement fixation test for syphilis in 1906. (Table 2) In his original report, extracts of beef heart were used as the antigen. The Wassermann test was the first serologic test for identification of syphilis. The antibody found in syphilitic sera was called reagin. Following Wassermann’s seminal paper, serologic tests for syphilis (STS) were used in screening the population as part of public health efforts to control syphilis. With mobilization of the Armed Forces for World War II, millions of STS were performed on military inductees. The most commonly used STS was the Venereal Disease Research Laboratory (VDRL) test employing a rapid sera flocculation procedure.
A.P. von Wassermann described a complement fixation test for syphilis in 1906. (Table 2) In his original report, extracts of beef heart were used as the antigen. The Wassermann test was the first serologic test for identification of syphilis. The antibody found in syphilitic sera was called reagin. Following Wassermann’s seminal paper, serologic tests for syphilis (STS) were used in screening the population as part of public health efforts to control syphilis. With mobilization of the Armed Forces for World War II, millions of STS were performed on military inductees. The most commonly used STS was the Venereal Disease Research Laboratory (VDRL) test employing a rapid sera flocculation procedure.
| Table 2 ANTICARDIOLIPIN ANTIBODIES: EVOLUTION OF TESTING | ||
| 1906 | A. P. von Wassermann | Reagin |
| 1930s | Venereal Disease Research Laboratory | VDRL |
| 1941 | Margaret Pangborn | Cardiolipin |
| 1952 | J. Moore and C. Mohr | BFP-STS |
| 1983 | N. Harris | ACA: Solid Phase RIA |
| 1984 | Koike T et al., Norberg et al. | ACA ELISA |
| LEGEND: VDRL: Venereal Disease Research Laboratory BFP-STS: Biologic False Positive Serologic Test for Syphilis |
With the wide spread use of the VDRL, an interesting clinical observation was reported in young women who were often identified as having a positive VDRL (usually associated with premarital testing required by State Boards of Health). However, these individuals had no history of sexually transmitted disease. This observation was also seen in the military where 50% of military personnel with positive VDRLs were found to have no clinical features of syphilis. The Biologic False Positive Serologic Test for Syphilis (BFP-STS) represented a major problem. As a result, the Treponema Pallidum Immobilization (TPI) test was introduced in the late 1940s. The TPI was much more specific for the diagnosis of syphilis.
![]() Also, in the 1940s, Margaret Pangborn found that alcoholic extracts of beef heart (source of antigen used in STS) contained a phospholipid which was later referred to as: cardiolipin.
Also, in the 1940s, Margaret Pangborn found that alcoholic extracts of beef heart (source of antigen used in STS) contained a phospholipid which was later referred to as: cardiolipin.
Following World War II, the phenomena of BFP-STS was studied by Joseph Moore and Charles Mohr (Johns Hopkins University). These investigators divided BFP-STS into acute and chronic categories. Patients with an acute BFP-STS had positive laboratory results which persisted for less than six months. Chronic BFP-STS by definition were those individuals who had positive results of greater than six months duration. Acute BFP-STS were usually identified in the context of infections (viral, bacterial, spirochetal, etc.). In contrast, patients with chronic BFP-STS typically had no history of infection. In their landmark study, Moore and Mohr found a high incidence of autoimmune disease (41.9%) in patients with chronic BFP-STS. Also, during their studies, Moore and Mohr observed a high incidence of BFP-STS in young women with clinical thrombocytopenia as well as thrombosis.
![]() In 1983, Nigel Harris and colleagues at Hammersmith Hospital (London, U.K.) developed a solid phase radioimmunoassay (RIA) to detect antibodies to cardiolipin (ACA). Their assay was reported to be approximately 400 times more sensitive than the conventional VDRL test. They subsequently reported ACA positive tests were correlated with both arterial and venous thromboembolic events, RFL, and thrombocytopenia. Subsequently, ELISAs to identify and quantitate ACA were developed by Koike et al. and Norberg et al. in 1984. A new clinical entity was proposed: “Anticardiolipin Syndrome” in 1985 by Graham Hughes (Rayne Institute, St. Thomas Hospital, London, U.K.).
In 1983, Nigel Harris and colleagues at Hammersmith Hospital (London, U.K.) developed a solid phase radioimmunoassay (RIA) to detect antibodies to cardiolipin (ACA). Their assay was reported to be approximately 400 times more sensitive than the conventional VDRL test. They subsequently reported ACA positive tests were correlated with both arterial and venous thromboembolic events, RFL, and thrombocytopenia. Subsequently, ELISAs to identify and quantitate ACA were developed by Koike et al. and Norberg et al. in 1984. A new clinical entity was proposed: “Anticardiolipin Syndrome” in 1985 by Graham Hughes (Rayne Institute, St. Thomas Hospital, London, U.K.).
ACA: Antigenic Targets
Antiphospholipid antibodies were initially thought to be a family of immunoglobulins which recognized anionic phospholipids (e.g., phosphatidylserine [PS], phosphatidic acid [PA], and cardiolipin [CL]. LA were also recognized as a member of the APA family.
| Table 3 SHORT CONSENSUS REPEAT (SCR) FAMILY | |
| COMPLEMENT FACTORS | OTHER PROTEINS |
| C4BP | Factor XIII |
| DAF | D-Selectin |
| Factor B | E-Selectin |
| Factor C | G-Selectin |
| Factor H | ß2 GPI |
| DAF = Decay Accelerating Factor |
![]() In 1990, a major breakthrough was independently reported by three groups of investigators. Their studies found the conventional ACA ELISA did not detect antibodies directed at cardiolipin. In fact, the antigenic target was a plasma protein: ß2 Glycoprotein I (ß2 GPI). (Figure 1) ß2 GPI is a physiologic anticoagulant found in plasma in a concentration of 200 µg/ml. Approximately 40% of ß2 GPI is associated with lipoproteins of various classes. ß2 GPI is a member of the Short Consensus Repeat (SCR) family of proteins. (Table 3) ß2 GPI inhibits the contact phase of coagulation, the prothrombinase reaction (conversion of prothrombin to thrombin) and ADP-induced platelet aggregation. Although ß2 GPI is an anticoagulant, hereditary deficiency of this protein does not lead to venous or arterial thromboembolic events. ß2 GPI is a single polypeptide chain composed of 326 amino acids. There are five homologous motifs of approximately 60 amino acids with highly conserved cysteines, prolines and tryptophans. These motifs are designated as SCRs. Because of their structure, they are often referred to as sushi domains.
In 1990, a major breakthrough was independently reported by three groups of investigators. Their studies found the conventional ACA ELISA did not detect antibodies directed at cardiolipin. In fact, the antigenic target was a plasma protein: ß2 Glycoprotein I (ß2 GPI). (Figure 1) ß2 GPI is a physiologic anticoagulant found in plasma in a concentration of 200 µg/ml. Approximately 40% of ß2 GPI is associated with lipoproteins of various classes. ß2 GPI is a member of the Short Consensus Repeat (SCR) family of proteins. (Table 3) ß2 GPI inhibits the contact phase of coagulation, the prothrombinase reaction (conversion of prothrombin to thrombin) and ADP-induced platelet aggregation. Although ß2 GPI is an anticoagulant, hereditary deficiency of this protein does not lead to venous or arterial thromboembolic events. ß2 GPI is a single polypeptide chain composed of 326 amino acids. There are five homologous motifs of approximately 60 amino acids with highly conserved cysteines, prolines and tryptophans. These motifs are designated as SCRs. Because of their structure, they are often referred to as sushi domains.
| Figure 1 STRUCTURE ß2 GLYCOPROTEIN I | ||
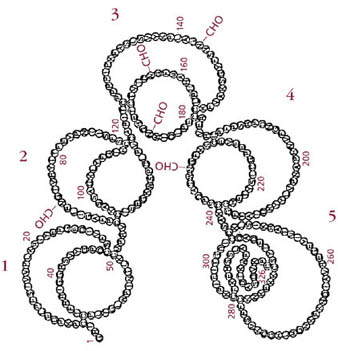 | ||
| LEGEND: ß2 GPI is a single polypeptide chain of 326 amino acids with five oligosaccharide attachment sites. There are five homologous motifs of 60 amino acids (Sushi domains). The fifth domain contains 82 amino acid residues [Sushi domains are numbered 1 – 5].Koike T et al. Lupus 7, Suppl 2, S14-S17, 1998. |
ß2 GPI in most cases represents the antigenic target in ACA ELISAs which utilize highly sensitive microtiter plates. (Figure 2) The ACA ELISAs used in the majority of laboratories detect antibodies to ß2 GPI. Anti-ß2 GPI antibodies are the dominant autoantibody in APA positive sera from patients with APS. The majority of anti-ß2 GPI antibodies bind to domains 1 or 4 of ß2 GPI. Commercial ELISA assays are now available for detection of antibodies to ß2 GPI. These ELISA kits use “sensitive” polystyrene microtiter plates which have been either oxidized or irradiated. Phospholipids are not used in these assay systems.
| Figure 2 ANTICARDIOLIPIN (ACA) ELISAs | ||
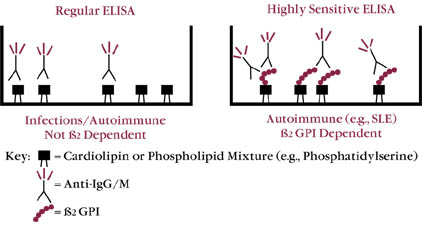 | ||
| LEGEND: The first generation of ACA ELISA assays detected autoimmune as well as alloimmune (seen in infectious conditions) antibodies. Alloimmune antibodies tended to be transient and not related to complications seen with autoimmune antibodies (i.e., RFL, arterial and venous thrombosis). |
The commercially available ACA ELISAs utilize cardiolipin or a mixture of anionic phospholipids (PS, CL) to coat the microtiter plates. In these assay systems, the ß2 GPI is contributed by the patient plasma (or ß2 GPI found in the blocking reagent). The conventional ACA is a more sensitive test in detecting the presence of antibodies to phospholipids (true antiphospholipid antibodies as seen in patients with infections [e.g., syphilis, etc.]) than are the newer anti-ß2 GPI assays. Commercial ACAs may detect both infection-related and autoimmune-related antibodies. The latter group of antibodies which consist principally of anti-ß2 GPI are of the most clinical significance. Typically, infection-related positive ACAs are transient. (Table 4)
| Table 4 ANTICARDIOLIPIN ANTIBODIES (ACA) | ||
| AUTOIMMUNE | ALLOIMMUNE* | |
| Cofactor | ß2 GPI | No |
| ß2 GPI Inhibitory | No | Yes |
| Clinical | VT/AT/RFL | Hx of Infections |
| Heat Inactivation | No | Yes |
| Tween 20 Enhances | Yes | No |
| LEGEND: VT = Venous Thrombosis, AT = Arterial Thrombosis, RFL = Recurrent Fetal Loss, Hx = History*Alloimmune – typical in infections. Will result in transient positive ACA. On repeat testing after 8 – 10 weeks will be negative in most cases. |
Reporting Results of ACA Elisa
The availability of isotype specific reference sera (IgG, IgM, IgA) has improved ACA ELISA testing. The use of these reference sera allows laboratories to semiquantitate the level of positivity. Isotype concentrations are expressed as GPL, MPL, or APL units as defined by Nigel Harris and colleagues. One unit represents the binding activity of 1 µg/ml of affinity purified ACA.
Utilizing the Harris reference sera, most laboratories report results as low positive >10 to <20 units, medium positive >20 to <80 units, and high positive >80 units. however, there remains some controversy regarding stratification of ACA results. many laboratories report negative, low positive, positive, high positive. other laboratories use multiples of the mean. ACA results should not be reported as standard deviations. unfortunately, the interlaboratory consistency of ACA results is poor. proficiency testing studies have demonstrated widely variable results between commercially-available ACA elisas.
In most cases, clinicians order IgG and IgM isotypes when utilizing the ACA ELISA. Interpretation of positive results may be compromised because of differences between various commercially-available ELISA assays. As previously noted, interlaboratory proficiency testing programs have repeatedly highlighted this problem. In the situation where the patient appears to have the clinical findings consistent with APS and negative LA/ACA testing for IgG and IgM isotypes, it is appropriate to request IgA ACA testing. In a relatively small subset of patients, IgA ACA may be the only positive laboratory result.
The clinical application of ACA results also depends upon demonstrating persistent ACA positivity. (Figure 3) An initial positive result should be followed up with another test eight to ten weeks later. Utilizing this approach, the transiently positive (infection-related) ACA will be identified. As noted above, in order to establish the diagnosis of APS, persistent positivity of ACA results and appropriately identified clinical complications are required to satisfy the definition of APS.
| Figure 3 ACA | ||
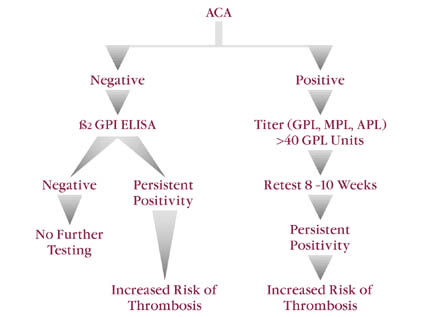 | ||
| LEGEND:Approximately 20% of patients with clinical thromboembolic events will have negative ACA test results and positivity for anti ß2-GPI. Anti-ß2 GPI ELISAs are less sensitive than ACAs but more specific with respect to identifying patients at risk for thrombosis.Antiphosphatidylserine testing is not used as frequently as ACA. However, anti PS appears to have a higher degree of sensitivity when compared to the standard ACA ELISA system. |
Levels of IgG ACA greater than 40 units have been linked to an increased risk of both venous and arterial thromboembolic disease. However, a recent study suggests the presence of persistent positivity (regardless of titer) identifies patients at risk of arterial thromboembolic events (Tuhrim et al.).
Anti-ß2 GPI ELISA
With the recognition of ß2 GPI as being the antigenic target in patients with autoimmune ACA, commercial ELISA assays for anti-ß2 GPI are now available. These assays employ high sensitivity microtiter plates (irradiated/oxidized). The high sensitivity plates simulate the in vivo activation of cellular membranes with exposure of phosphatidylserine from the inner leaflet of the cellular membrane. The density of ß2 GPI on the microtiter plate is increased when the plates are oxidized. (Figure 4) The increased density allows for bivalent bonding of anti-ß2 GPI antibodies. The persistence of positive anti-ß2 GPI identifies patients at higher risk of thromboembolic events (this assay is more specific in identifying patients at risk than a positive conventional ACA). (Table 5)
| Figure 4 ANTI-ß2 GPI ELISA | ||
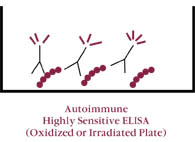 | ||
| LEGEND: Utilizing highly sensitive microtiter plates, there is greater “density” of ß2 GPI binding. The increased density of ß2 GPI allows for bivalent bonding of antibodies against this protein. The “clustering” of anti-ß2 GPI on microtiter plates is thought to be analogous to bivalent binding of ß2 GPI to activated cellular membranes in vivo (e.g., activated platelets, activated endothelial cells, circulating platelet microparticles, etc.). When ELISAs for anti-ß2 GPI are compared to ACA testing, they are found to have greater specificity and predictive value for clinical complications. However, ACA testing has greater sensitivity when compared to anti-ß2 GPI.Sanmarco et al. Lab Clin Med 1997 |
Antibodies to ß2 GPI are also important in the pathogenesis of human atherosclerosis. In a recent study by George et al. (1999) utilizing immunohistologic studies, the deposition of ß2 GPI in atherosclerotic plaque was demonstrated. ß2 GPI may also play a role in the arterial plaque deposition of oxidized low density lipoproteins (ox-LDL). Antioxidant vitamins E and C have been suggested as therapeutic options in lowering plasma levels of oxidized LDL. Also, urinary isoprostanes are markedly decreased when patients are treated with vitamins E and C (Pratico et al.).
| Table 5 ANTIPHOSPHOLIPID SYNDROME (APS): ANTI-ß2 GPI/ACA TESTING | ||
| Anti-ß2 GPI | ACA | |
| Specificity | 98% | 54% |
| Sensitivity | 54% | 87% |
| Predictive Value* | 87.5% | 34.6% |
| *(Thromboembolic Events)LEGEND: IgG anti-ß2 GPI have higher specificity and greater positive predictive value when compared with ACA testing. IgG anti-ß2 GPI levels are highly correlated with ACA found in autoimmune diseases. In contrast, true anticardiolipin antibodies (ACA) are found in higher prevalence in patients with infectious diseases (40%). Anti-ß2 GPI antibodies have high specificity and are useful in evaluation of patients at risk for APS. Because of their low sensitivity, their detection needs to be associated with ACA testing.Sanmarco et al. J Lab Clin Med 129:499-506, 1997 |
Summary
Traditional ACA testing developed in the 1980s continues in laboratories today. However, recent advances have resulted in recognition of antigenic targets for APA. The two most commonly encountered antibodies are directed against ß2 GPI and prothrombin. Antibodies which recognize ß2 GPI have been associated with venous and arterial thromboembolic events. Incorporation of tests for antibodies to ß2 GPI is important in identifying patients with APS and also correctly categorizing which patients are at greatest risk of thromboembolic events.

