Changes in Virus Detection Protocols
Virus cultures are algorithm-based detection procedures used to isolate and identify viral agents associated with human disease. Virus cultures can be used to detect a single agent (herpes simplex virus, varicella-zoster virus, cytomegalovirus, etc.) or they can be used to detect a broad range of agents. Broad-range or comprehensive virus detection is traditionally done by cell culture inoculation, direct observation of cultures for virus-specific cytopathic effect, and fluorescent antibody staining. These procedures allow the laboratory to detect most adenoviruses and enteroviruses, herpes simplex virus, influenza A and B, measles, mumps, parainfluenza 1, 2, and 3, respiratory syncytial virus, rhinovirus, and varicella-zoster virus in a variety of clinical specimens. Culture-based detection is extremely useful but this procedure has several limitations for the practicing physician. Cell culture isolation and identification can take days to weeks and some viruses causing human infection do not grow in cell culture. In addition, some culturable viruses may not be viable when they arrive in the laboratory.
Direct fluorescent antibody (DFA) methods were developed in the 1970s. These procedures were less expensive than culture and they allowed the laboratory to detect certain viral agents within hours instead of days. The specimen collection and transport requirements for DFA were less stringent than culture because DFA methods did not require virus viability. Unfortunately, DFA methods cannot detect all culturable viruses, they are less sensitive than culture, and they cannot be used with all specimen types. Despite these limitations, DFA methods have played an important role in virus detection by providing faster turnaround times and good specificity.
The accuracy and speed of virus detection has been dramatically improved through the introduction of polymerase chain reaction (PCR) testing and more recently, real-time PCR detection. PCR is more sensitive than culture or DFA, it is highly specific, and can be used with the same specimen types as culture. Because PCR methods do not depend upon virus viability, specimen handling requirements for PCR are less stringent than for culture.
In an effort to provide the most sensitive, specific, and rapid testing possible, Warde Medical Laboratory has replaced the comprehensive viral culture and many of the single test cultures with simplified virus testing protocols. Virus culture is still available (see below) and the new Comprehensive Virus Detection (CVD, test code 68-00900) protocol provides faster and more sensitive virus detection for a broader range of viral agents.
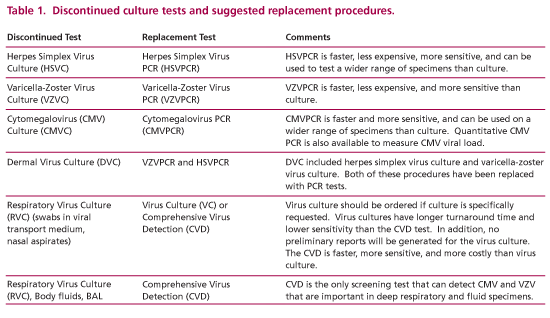
Single-agent cultures. To enhance the sensitivity of virus testing procedures, single agent cultures (e.g., herpes simplex, varicella-zoster virus, and cytomegalovirus) have been eliminated and replaced with PCR tests. PCR procedures are more sensitive and less expensive than virus culture and they provide test results significantly faster than culture procedures. Specimen handling for PCR is less stringent than culture thereby improving the accuracy and convenience of the testing process. Discontinued culture tests and the recommended test substitutions are summarized in Table 1.
Virus Culture (VC). Physicians sometimes order culture procedures for medico-legal purposes (i.e., alleged sexual assault) or to obtain an isolate for antiviral susceptibility testing. The VC procedure is appropriate for both purposes. However, virus culture has limitations when used for routine clinical purposes (Table 2). Virus cultures are slow (up to 14 days to a negative result) and no preliminary reports are generated with this procedure. Virus cultures can be problematic because some clinical specimens (e.g., urine, bronchial washes, and body fluids) can contain substances that are toxic to the cell monolayers. Virus cultures also have trouble detecting multiple viral agents in a single specimen because rapidly growing viruses will destroy the cells before slower growing viruses can be detected. PCR methods are unaffected by the presence of other nucleic acids and the use of PCR methods can enhance the detection of co-infections.
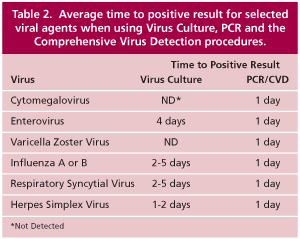
The virus culture offered by Warde Medical Laboratory cannot reliably detect varicella zoster virus or cytomegalovirus because special culture procedures are required to detect these agents in a timely manner. Virus cultures can be performed on limited specimen types. Virus cultures are not recommended for cerebrospinal fluid (CSF), blood, serum, bone marrow, and body fluid, and fluid aspirate specimens and bronchial washes. Urine specimens are acceptable only for mumps and measles detection. Recommended alternative tests are shown in Table 1. Viruses that can be detected by culture include most adenoviruses, most enteroviruses, herpes simplex virus, influenza A and B, measles virus, mumps virus, respiratory syncytial virus, rhinovirus, and parainfluenza types 1, 2, and 3.
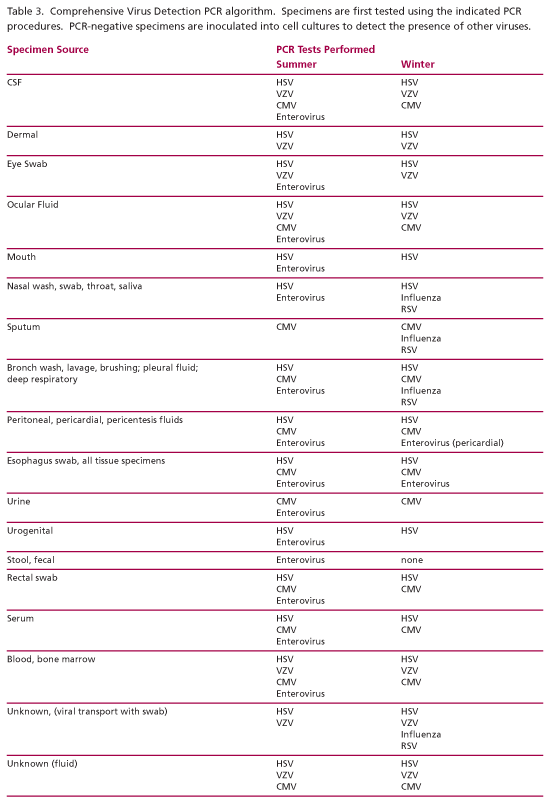
Comprehensive Virus Detection (CVD). The CVD procedure is designed to provide rapid results and broad virus coverage. This procedure utilizes algorithm-based (season- and specimen source-driven) PCR testing (Table 3) followed by traditional culture if the PCR testing is negative. Culture allows the laboratory to detect viruses that cannot be detected by PCR at this time. The use of PCR testing expands the number of agents that can be detected including the unculturable enteroviruses, varicella-zoster virus, and cytomegalovirus. PCR testing can detect viruses that are no longer viable including viruses in older, crusted lesions and in specimens that have not been refrigerated. The increased sensitivity, ability to detect more viruses, and the improved turnaround times associated with PCR testing can improve the clinical relevance of the virus detection result.
For More Information. Contact the Virology Laboratory (734-214-0350) for information regarding specimen collection, stability, sources, and testing status. Dr. Wiedbrauk (734-214-0300) is available to answer questions regarding test selection and interpretation.