Consensus Guidelines for Evaluating Monoclonal Gammopathies
Last May, new National Consensus Guidelines for the Clinical and Laboratory Evaluation of Patients with Monoclonal Gammopathies were adopted. The Guidelines conference was co-sponsored by: The Clinical Center of the National Institutes of Health, The American Society of Hematology, The American College of Rheumatology, and The College of American Pathologists.
The conference focused on three questions:
- Which patients should be evaluated for the presence of a monoclonal (M) protein?
- What types of specimens should be studied, and how often should they be used to follow patients with M-proteins?
- Which laboratory methods should be used to detect, identify and follow M-proteins?
Which patients should be evaluated for the presence of an M-protein?
M-proteins are found in the serum and/or urine of patients with a wide variety of clinical conditions. The occurrence and clinical symptoms of these conditions vary widely (Table 1). For instance, approximately 13,000 new cases of multiple myeloma are reported annually in the United States, whereas, the estimated occurrence of monoclonal gammopathy of undetermined significance (MGUS) has been as high as 750,000. Patients with multiple myeloma have an average survival of 3 years, while individuals with MGUS have the average life expectancy for their age group (about 10 years). Clearly the significance of M-proteins varies widely and the mere presence of an M-protein must be evaluated in the clinical context of that patient.
| Conditions Associated with the presence of an M-protein | |
| CONDITION | CLINICAL FEATURES |
| Multiple Myeloma | Back pain, osteolytic lesions, fatigue, elevated sedimentation rate, nephrotic syndrome, infections, anemia, elevated calcium |
| Waldenström’s Macroglobulinemia | Fatigue, elevated sedimentation rate, dizziness, anemia, hyperviscosity |
| B-Lymphoproliferative Disorders | Fatigue, anemia, lymphadenopathy, lymphocytosis |
| Amyloyd (AL), Light Chain Deposition Disease | Nephrotic syndrome, congestive heart failure, carpal tunnel syndrome |
| Neuropathy and M-protein | Peripheral sensory &/or motor neuropathy |
| Monoclonal Gammopathy of Undetermined Significance (MGUS) | No symptoms related to the M-protein |
Traditionally, the size of the M-protein has been helpful in determining the significance for the patient. Typically, a large quantity of M-protein (greater than 2 G/dl) is more likely to be associated with myeloma, whereas, a small quantity of M-protein may be associated with MGUS. Yet, several important conditions associated with small quantities of M-proteins may be overlooked if these M-proteins are not recognized (Table 2). This is why it is important to use a logical sequence of testing and high quality laboratory methods to detect M-proteins.
| Conditions with Relatively Small Quantities of M-protein in Serum | |
| CONDITION | CLINICAL FEATURES |
| Light Chain Disease | 15% of cases of Multiple Myeloma are light chain disease |
| Amyloid (AL) | 20% have Multiple Myeloma or Waldenström’s Macroglobulinemia |
| Heavy Chain Disease | Alpha chain disease is rare in US Gamma chain disease is associated with B-lymphoproliferative disorders |
| M-protein Associated Neuropathy | Must do Immunofixiation of Serum to Rule Out |
| Cryoglobulin Type II | Associated with Hepatitis C |
| Solitary Plasmacytoma | Tiny or no M-protein in serum |
| B-lymphoproliferative Disorder | Often with overall gamma suppression |
| MGUS | The most common cause of serum M-proteins |
Due to the high prevalence of MGUS, it is not useful to screen patients who do not have signs or symptoms consistent with the other conditions listed in tables 1 and 2 with serum and urine protein electrophoresis. In order for a positive result to have a significant predictive value for the individual patient, serum and urine protein evaluations must be performed selectively. The degree of clinical suspicion is also useful in determining how far to proceed with the testing process.
What types of specimens should be studied, and how often should they be used to follow patients with M-proteins?
What evaluations should be made first?
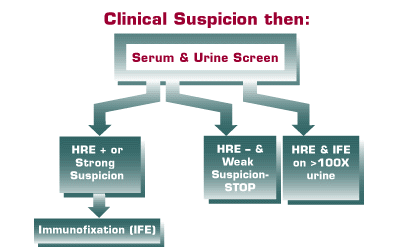
The initial evaluation of a patient with clinical features suggesting the presence of an M-protein should include a serum and urine electrophoresis using high-resolution methods (Figure 1). It is important to include both urine and serum in this initial evaluation, because in some patients the M-protein will only be located in one or the other fluid. Alternatively, in some cases, only a small amount of M-protein will be found in the serum while a massive amount of monoclonal free light chain (Bence Jones protein) will be present in the urine.
 |
What to do after the screen?
If the serum protein electrophoresis is negative, and there is a low index of suspicion, one may wish to look for other causes of the symptoms before performing an immunofixation (IFE). However, if the clinical suspicion is high, an IFE should be requested even when no M-protein is seen on the screening electrophoresis. If an M-protein is found on the screening electrophoresis, an IFE must be performed to identify the M-protein. This identification serves several purposes. First, it rules out a false positive due to some other protein that may mimic an M-protein on serum protein electrophoresis (Table 3). Second, since IFE is more sensitive than screening electrophoresis, it may identify a second M-protein (often a monoclonal free light chain that accompanies the intact molecule in the serum). Third, it identifies the M-protein for comparison with subsequent samples from the patient. If there is no change in the migration of the identified M-protein on subsequent samples, there is no need to perform further IFE studies. Repeat IFE studies are only needed when the electrophoretic migration changes on subsequent samples because this may indicate the development of a second clone, or the appearance of monoclonal free light chains in addition to the intact M-protein.
How are monoclonal free light chains detected?
| Protein that may mimic an M-protein | |
| Fibrinogen | |
| Transferrin Variant | |
| C3 Variant | |
| Beta-1 lipoprotein | |
| Latrogenic, eg. Urografin on capillary zone electrophoresis | |
| Latrogenic, eg. Urografin on capillary zone electrophoresis |
Although one may detect monoclonal free light chains (Bence Jones proteins) in the serum, they mainly are found in the urine. Their small size (20 kDa for monomers and 40 kDA for dimers) allows them to pass through the glomerular basement membrane. Thus, these smaller molecules are more quickly depleted from the serum than their counterpart intact M-proteins (160 kDa). Urine for evaluating the presence of monoclonal free light chain should be a 24 hour sample. False negatives are most likely to occur on spot samples of urine. Therefore, a negative result on a spot urine sample cannot be used to rule out the presence of monoclonal free light chains.
All urine samples suspected of containing an M-protein must be concentrated at least 100X by the laboratory and must be tested by both electrophoresis and IFE. False negatives may occur with dip sticks, sulfosalicylic acid tests and the old heat acid precipitation test. There is an incorrect general belief that the sulfosalicylic acid test is an adequate screen. The monoclonal gammopathy panel of experts was unanimous that it is not. All panel members reported seeing several cases of monoclonal free light chain missed by the sulfosalicylic acid method.
Once an M-protein has been identified, how should it be followed?
Depending on the population of patients selected for M-protein evaluation, most cases will be MGUS. However, one must rule out all of the relevant conditions listed in Tables 1 and 2 before classifying a patient as MGUS. For instance, congestive heart failure due to amyloid (AL) may be suspected if one detects a small quantity of lambda M-protein in the serum or urine. Once those conditions are ruled out, patients classified as MGUS need to have serum and urine quantifications of the M-protein only annually, assuming no change in the clinical or laboratory features. Kyle has found that about 20-25% of patients with MGUS followed for 20 -35 years will develop clinically significant B cell lymphoproliferative disorders including: myeloma, Waldenström’s macroglobulinemia, B cell leukemia and B cell lymphoma.
Patients with multiple myeloma and Waldenström’s macroglobulinemia who are receiving therapy need to be followed every 1-2 months with serum and urine electrophoresis. The laboratory needs to report the quantity of the M-protein as a guide to the effectiveness of ongoing therapy. It is wasteful to repeat the IFE on samples with no change in the migration of the M-protein by serum protein electrophoresis. A repeat IFE does not provide useful information. The panel was unanimous that the clinician needs to know what has happened to the quantity of the M-protein since the last evaluation, not to re-identify a known molecule.
How is serum M-protein measured?
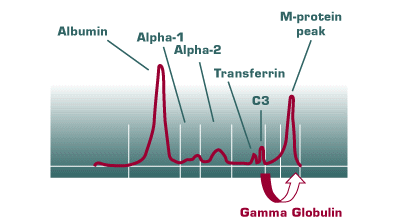
For serum, the best measurement of the M-protein is achieved by performing high-resolution electrophoresis and measuring the amount of protein below the spike (Figure 2). Sometimes, when the M-protein migrates in the beta region, this may be a problem if the quantity of the M-protein is small and it co-migrates with either transferrin or C3. In practice, however, most clinically significant M-proteins either migrate in the gamma region, or are large enough in quantity that the interference in measurement by transferrin or C3 is relatively trivial. For those few cases, however, quantification of the M-protein by measuring total IgA or IgM will provide adequate follow-up.
 |
How is urine M-protein measured?
For the urine, it is important to follow the monoclonal free light chains and not the intact M-protein immunoglobulin that may be found in the urine. The prognosis and threat to the kidney is mainly due to the amount of monoclonal free light chain and not due to the intact M-protein.
The panel agreed that the term “Bence Jones protein” has been somewhat confusing in this regard. Bence Jones protein only refers to the monoclonal free light chains, not to the intact monoclonal immunoglobulins. Because of this, the consensus panel recommends use of the more specific term “monoclonal free light chain” instead of the older term “Bence Jones protein”. The panel recognized that because of the long use of the term “Bence Jones” protein, it would be a slow process to remove it. However, the confusion caused by mistaking intact M-proteins with monoclonal free light chains is of enough significance to warrant this effort.
Monoclonal free light chains may damage renal tubules, and are associated with amyloid (AL), as well as light chain deposition disease. To follow the quantity of monoclonal free light chain in the urine, obtain a 24 hour sample of urine. The laboratory needs to perform electrophoresis and determine the amount of protein associated with the monoclonal free light chain peak. If there has been any change in migration of the monoclonal free light chain, the laboratory needs to perform IFE to determine if a new peak is another form of monoclonal free light chain (dimer, tetramer) or a new intact M-protein peak. Results are expressed as grams of monoclonal free light chain/24hours.
Which laboratory methods should be used to detect, identify and follow M-proteins?
The panel was unanimous that the laboratory should use a high-resolution electrophoretic method in the initial evaluation of serum and urine. A high-resolution method is one that permits a crisp separation of transferrin and C3 in the beta region. This gives the interpreter the best view of subtle, but potentially clinically significant M-proteins.
High-resolution gels are necessary.
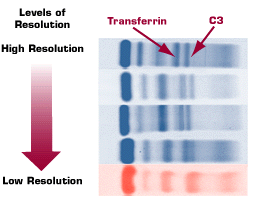 |
There is a wide variation in the quality of electrophoretic gels available. At Warde Medical Laboratory we use the highest quality capillary zone and agarose gel electrophoresis methods, currently approved by the FDA, for the evaluation of M-proteins. An example of the degrees of resolution from available gel-based methods is shown in Figure 3. The importance of using high-resolution electrophoresis methods was demonstrated on a national level in a College of American Pathologists (CAP) Survey Sample. Less than one-third of laboratories using a low-resolution electrophoretic system could detect a subtle, but potentially clinically significant, M-protein in the CAP Survey, while over 95% of laboratories using high-resolution methods were able to detect this M-protein.
Capillary Zone Electrophoresis
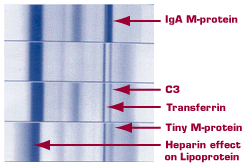 |
In addition to gel-based methods, capillary zone electrophoresis is also available in a high-resolution format. The crisp separation of the beta region components by high-resolution capillary zone electrophoresis is evident in the examples shown in Figure 4. Our laboratory uses exclusively high-resolution capillary zone and high-resolution gel-based methods in our evaluation of serum and urine for M-proteins.
How is urine evaluated?
All urine must be evaluated by both high-resolution electrophoresis and by IFE on the initial evaluation for the presence of an M-protein. The urine must be concentrated at least 100X prior to study. It is important to do both tests initially to be certain that an obvious M-protein seen on the high-resolution electrophoresis of the urine is not missed due to a technical error, or to an antigen excess effect. IFE must be able to detect the presence of kappa and lambda monoclonal free light chains. When monoclonal free ligth chains are identified, they must be quantified on 24 hour samples to follow the patients.
The presence of a monoclonal free light chain in the urine is more ominous and uncommon than the presence of a small M-protein in the serum. This is because the tubules are capable of reabsorbing up to 1 gram of protein in 24 hours. Before the monoclonal free light chains are detected in the urine, they must be present in a relatively large amount to exceed the tubular reabsorptive capacity. Therefore, when one detects a monoclonal free light chain in the urine, one must be particularly mindful of the more subtle conditions such as amyloid (AL) and light chain deposition disease that may be associated with those M-proteins.
Summary
This review of the 1998 Consensus Guidelines for the Work up of Monoclonal Gammopathies has summarized the major features of selecting patients who should be tested, optimal ways to follow patients with M-proteins, and laboratory methods that should be used to evaluate these individuals. Four articles about this conference are published in the February, 1999 edition of Archives of Pathology and Laboratory Medicine.
If there are questions about the use of the Guidelines, or the evaluation of patients with M-proteins feel free to call Warde Medical Laboratory at 800 876-6522 for advice, or email Client Services with your concerns.

