Detection of Prenatal Drug Abuse in Meconium
Introduction
Alcohol and drug abuse in pregnancy is a significant social and medical issue. The 2009 National Survey on Drug Use and Health (NSDUH) estimated that approximately 21.8 million Americans aged 12 or older were using illicit (illegal) drugs (marijuana, psychotherapeutics, cocaine, hallucinogens, inhalants or heroin). Figure 1 shows the distribution of drug use a month prior to the national survey. According to this survey, overall the rate of illicit drug use increased by 8% in the period 2008-2009. The survey also showed that illicit drug usage varied according to age (Figure 2). Females constitute approximately 30% of all the substance addicted population in the United States in the year 2008 alone (1). Among women, the survey showed that there is also a relationship between illicit drug use, age and whether they are pregnant or not as shown in Table 1.
The same survey also showed that drug abuse varied significantly by race or ethnic groups as shown in Figure 3.
Together the data suggest that a significant percentage of the American population including pregnant women (Table 1) is involved with illegal drug use.
Figure 1. Past months abuse of specific drugs among persons aged 12 and older.
| 1 Illicit Drugs (marijuana, cocaine, heroin, hallucinogens,inhalants, or prescription type psychotherapeutics used non-medically). This includes the abuse of more than one drug by an individual. |
(Source: http://www.oas.samhsa.gov/nsduhLatest.htm) 1.
Figure 2. Age grouping and illicit drug use among persons aged 12 or older
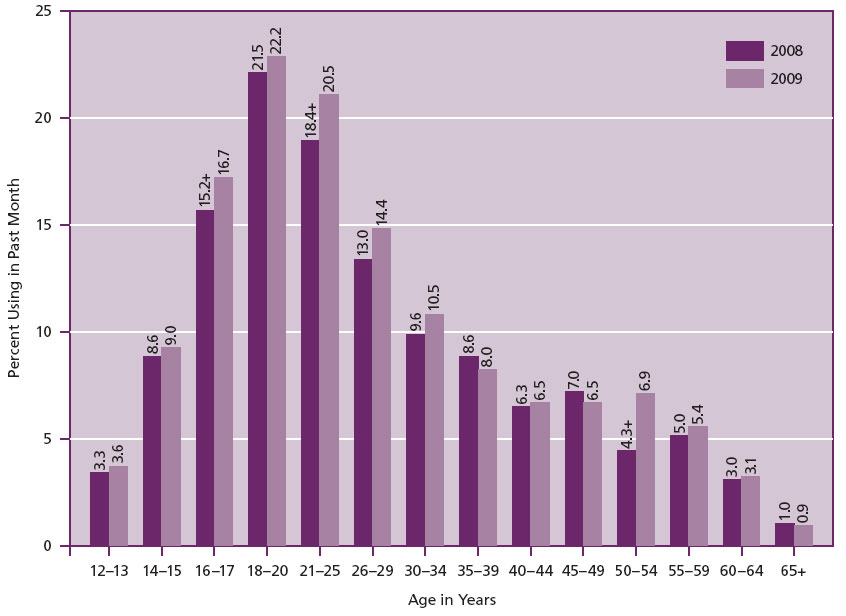
(Source: http://www.oas.samhsa.gov/nsduhLatest.htm).
Figure 3. Ethnic distribution of drug use in the USA
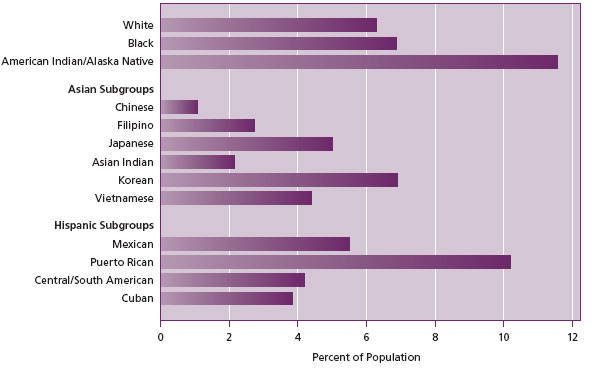
(Source: http://www.oas.samhsa.gov/nsduhLatest.htm).
Table 1. Age of women and illicit drug use in the 2008-2009 NSDUH survey
| Age range | Pregnant (%) | Non-pregnant (%) |
| 15–17 | 15.8 | 13.0 |
| 18–25 | 7.1 | 16.8 |
| 26–44 | 2.2 | 7.6 |
| 15–44 | 4.5 | 10.6 |
Effects of Prenatal Drug Abuse
Prenatal drug abuse undoubtedly poses both medical and economic problems to society. Babies exposed to in utero drugs of abuse such as opioids may suffer multifunctional disorders that frequently involve the central nervous system (CNS), gastrointestinal system (GI), autonomic and respiratory systems. This condition has been called the neonatal abstinence syndrome (NAS) because these problems are due to a sudden withdrawal of the drug after birth. According to a Virginia area study the average cost of infants diagnosed with this syndrome is $36,000 relative to $2,000 for a normal baby (2). The potential adverse effects (Table 2) on the fetus of in utero exposure to drugs of abuse are well recognized (3). In order that appropriate care and treatment is given to both mother and the newborn, it is imperative that early and reliable diagnosis is made to improve developmental outcomes for children born to drug-abuse mothers.
Table 2. Effects of drug abuse on the newborn
| Drug | Findings of Effects on Newborn |
| THC (Cannabinoids) | • Poor performance on various lQ tests (e.g. performance in short-term memory and verbal reasoning subscales) observed around 3 years of age • Behavioral problems |
| Cocaine | • Low birth weight, length and head circumference • Premature rupture of membrane • Placental abruption result in maternal and/or fetal death • Language deficits (expressive and receptive language) |
| Amphetamine | • Fetal growth restrictions • Increase the rate of premature delivery and placental abruption • Aggressive and hyperactive behavior • Lower scores on measures of attention, memory, long term spatial memory, visual motor integration |
| Opioids | • Intrauterine growth retardation and pre-term deliveries • Neonatal abstinence syndrome (NAS) • Short attention span, hyperactivity, sleep disturbances, perception and memory difficulties |
Diagnosis
Because it is not recommended to screen all mothers for illicit drug abuse (4, 5), physicians follow set guidelines to identify newborns qualified for drug testing. Among the initial indicators triggering an investigation into prenatal drug abuse are (6):
- the mothers past history (checkups before delivery)
- unexplained premature birth or labor
- low birth weight
- intracranial bleeding
- small head circumference
- newborn signs of withdrawal (seizures, irritability, hypertonia, sneezing, yawning, sweating, GI dysfunction, feeding problems and muscle rigidity).
- mothers with certain diseases such as syphilis, gonorrhea and hepatitis B are also candidates for drug testing
- Self report
If there is sufficient reason to follow up these observations, in conjunction with maternal self report (usually unreliable for fear of losing custody or otherwise guilt), physicians or care providers request a drug test of the newborn, mother, or both, from the medical toxicology laboratory. The most accurate method for detecting prenatal drug abuse is testing of the newborn.
Sample Types for Drug of Abuse Testing in the Newborn
Bodily fluids or tissues (matrices) required for prenatal drug of abuse testing of the newborn include:
- Urine (neonatal or maternal)
- Hair (neonatal or maternal)
- Umbilical cord
- Meconium (neonatal)
- Nails (neonatal)
Urine and neonatal hair will detect more recent prenatal drug abuse (2 to 3 days). However these matrices have their disadvantages. It is usually difficult to collect and get sufficient sample to perform drug testing especially on neonate urine. Additionally the half life of most drugs in urine is very short (2 to 3 days). Hair testing is becoming increasingly popular but has some disadvantages including the contention that there is a bias regarding hair color (some drugs tend to accumulate more in dark hair than for other hair colors) (7). Cosmetic treatment of hair can also interfere with the natural state of the hair and consequently its drug retention. Hair testing is generally more demanding than urine or meconium testing because among others, control samples are difficult to establish.
Meconium
Meconium is the fecal material passed by the newborn within the first three days of birth. The color is generally dark-green due to the presence of bile pigmentation. It begins to form between 12 and 16 weeks (second and third trimester) of gestation until birth and therefore it can provide a history of in utero drug exposure.
Meconium is formed from:
- swallowed amniotic fluid
- intestinal secretions (e.g. bile)
- and cellular debris.
At full term fetal meconium consists of:
- water
- mucopolysaccharides
- epithelial cells
- cholesterol
- sterol precursors
- proteins and lipids
- bile acids and bile salts (excreted by the beginning of the second trimester)
- enzymes
- blood group substances
- squamous cells and
- vernix caseosa (sebum and desquamated cells from fetal skin).
Advantages and disadvantages of Meconium Testing
Meconium is relatively easy to collect in a non-invasive manner. Most drugs are stabile in meconium at room temperature for up to one week. However for most clinical laboratories meconium, unlike urine, is a difficult, labor intensive sample for analysis. The small sample size, lack of homogeneity, different metabolic profiles and the requirement for low limits of detection present analytical challenges for drug testing.
Analytical Methods for Meconium Drug Testing
An accurate and early identification of babies exposed to drugs of abuse is critical for their future development and mother’s care. The testing methodology for such identifications must, therefore, be sensitive enough (low limit of detection) to pick up any drugs present in the sample. Secondly, the method must be specific to pick up the correct drug. The two common approaches to meconium drug testing are an initial screening (usually by immunoassay) followed by a confirmation of all immunoassay presumptive positives (usually by chromatography).
Immunoassays
Various immunological methods have been developed; in most cases targeted at a single drug and or its metabolite(s) in urine. Some of these have been adapted and widely used for the initial rapid screening of a limited number of drugs of abuse in various matrices such as serum and meconium. Because immunological methods are based on antibody/ antigen interactions, they tend to be relatively nonspecific for some drugs and therefore subject to interferences (8) which can give false positive (e.g. immunoassay response to unrelated compounds) or negative results (e.g. a wrong target drug leading to insufficient response). Immunoassays are also difficult to quickly adapt for the detection of new drugs in meconium because they were originally designed for drug testing in urine samples. Recently though, immunoassay sensitivities have been improved by the development of a meconium specific assay (9). Nevertheless, immunological testing is fast and less labor intensive, hence their utility in drug screening as the first line of testing toward identifying drugs in patient samples.
Chromatographic Methods
Chromatographic methods are generally used to confirm presumptive positive immunological screen results (see immunoassays above). These methodologies are more sensitive and specific but more labor intensive and require special expertise. Consequently, they have a long turn around time and therefore not suitable for screening. However, the advent of liquid chromatography coupled to tandem mass spectrometric detectors (LC-MS/MS) has made drug screening by chromatography feasible. Due to its high resolution, the mass spectrometer can detect a broad spectrum of drugs and their metabolites in one run. If the method is rigorously validated, interferences, if present, can be eliminated most of the time. LC-MS/MS can therefore be used to screen for multiple drugs and their metabolites in a patient sample (10, 11, 12).
Meconium Testing at Warde Medical Laboratory and Methodology.
The meconium drug screen panel at Warde Laboratory includes:
- opiates (morphine, hydromorphone, oxycodone)
- cocaine and its metabolites (benzoyle ecgonine and m-hydroxybenzoyl ecgonine)
- methadone
- phencyclidine
- amphetamine
- Metamphetamine
- THC
It is recommended that meconium samples collected at different times for a baby (e.g. a preterm baby where several collections are required in order to get sufficient meconium) be pooled together before being sent to the laboratory for testing.
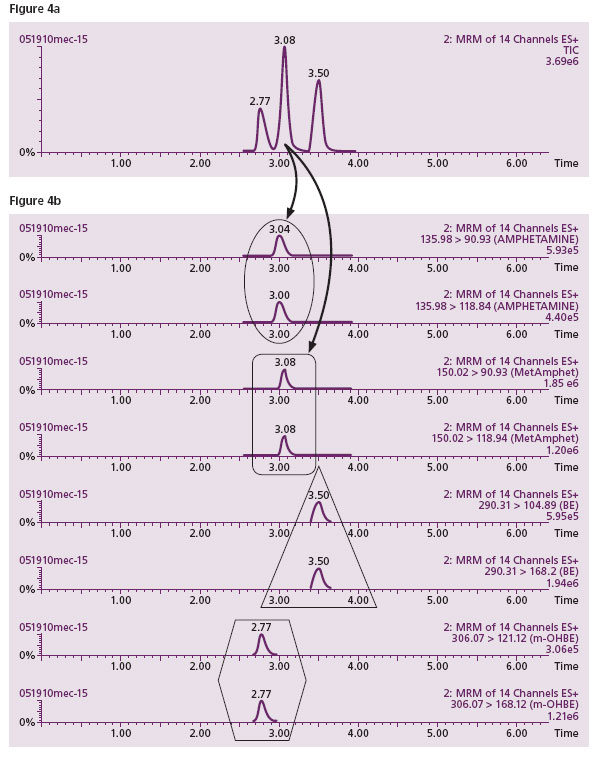
Illicit drug testing of meconium samples at Warde Medical Laboratory utilizes screening by LC-MS/MS, a methodology which has been in routine operation in our laboratory for more than four years. This combines the sensitivity and selectivity required to accurately identify drugs in meconium. The positive cutoff for all drugs is 20 ng/mL except amphetamine and methamphetamine which have a cutoff of 40 ng/mL. THC is screened by immunoassay at the present time. Figure 4 shows a typical LC-MS/MS screen for amphetamine, methamphetamine, benzoylecogonine (BE) and the meconium specific metabolite of cocaine, m-hydroxybenzoylecgonine (m-OHBE). Figure 4a shows three peaks separated by HPLC depicting ideally three drugs eluting at different times during a chromatographic separation. Figure 4b (the MS/MS detector) shows that there are actually four drugs (two identifier transitions specific for each drug) because it is revealed that the peak eluting at time 3.08 minutes actually has two different drugs (amphetamine and methamphetamine shown by the four specific transitions) under the peak. This is shown by the similar times of this peak/transitions in both Figures 4a and 4b. This type of interference (a weakness of both immunoassays and HPLC) is identified and eliminated if need be, by LC-MS/MS. The method is sensitive and specific for the drug or drugs present in an individual.
Figure 4a. A typical HPLC separation of drugs shows just three peaks. This seems to indicate only 3 drugs present in the sample.
Figure 4b. However, when the contents of the individual HPLC peaks were subjected to mass spectrometric analysis, the middle peak revealed a co- migration of two drugs (top 4 lanes in 4b. where each drug is represented by two specific fragments in two lanes). The top two lanes (oval) show two specific fragments generated from amphetamine, the parent molecule with mass 135.98. The next two lanes (Rectangle) display fragments generated from the second parent molecule, methamphetamine with a mass of 150.02. The first and third peaks from HPLC indicate two cocaine specific metabolites (triangle and hexagon in the bottom four rows).
Summary
Reliability of drug testing on meconium samples by immunoassay alone is subject to substantial interferences without a confirmation by an alternate method. Screening by LC-MS/MS has the advantage of simultaneously detecting a broad spectrum of drugs and their metabolites without the kind of interferences experienced with immunoassays. Warde Medical Laboratory recommends meconium drug screen by LC-MS/MS which is more sensitive and specific for drugs of interest in this matrix.
References
- http://www.oas.samhsa.gov/nsduhLatest.htm
- Baxter FR., Nerhood R., Chaffin DG. Characterization of babies discharged from Cabell Huntington Hospital during the calendar year 2005 with the diagnosis of neonatal abstinence syndrome. West Virginia Medical Journal 2009;105:16-21.
- Schempf AH. Illicit Drug Use and Neonatal Outcomes. A Critical Review. Obstetrical & Gynecological Survey. 2007;62: 749-757.
- Committee on Substance abuse. Drug-exposed infants. Pediatrics 1995; 96: 364-6.
- Christian JT., Knisely JS., Dawson KS., et al. Comparison of questionnaire screening and urine toxicology for detection of pregnancy complicated by substance abuse. Obstet. Gynecol. 1992; 80:750-754.
- Ryan RM., Wagner CL., Schultz JM., Varley J., DiPreta J., Sherer DM., et al. Meconium analysis improves identification of intrauterine cocaine exposed infants. J Pediatr. 1994; 125: 435-40.
- Douglas ER., Diana GW., Gerald GK., Marc PA., Atsuhiro M., Carol ON., Chad RB., Mathew HS. The effect of hair color on the incorporation of codeine into human hair. J. Anal. Toxicology. 2003; 27: 545-551.
- Micheal EM, Michele MB, Micheal WS. False positive Tricyclic Antidepressant Drug Screen Results Leading to the Diagnosis of Carbamazepine Intoxication. Pediatrics 2000; 105: 1-3.
- Stephanie JM., Lindsay K., Miles M., Gwendolyn AM. J. Anal. Toxicology 2009; 33: 148- 154.
- Nigel WB., Micheal EF., Eyrun NE., Christopher EG., Maria P., Paul JT and Micheal JT. An investigation into the bias between LC-MS/MS and an Enzyme Multiplied Immunoassay Technique (EMIT) for the measurement of Mycophenolic Acid. Ther. Drug Monit. 2010; 32: 420-426.
- Sauvage FL., Picard N., Saint-Marcoux F., Gaulier JM., Lachatre G., Marquet P. General unknown screening procedure for the characterization of human drug metabolites in forensic toxicology: applications and constraints. J. Spec. Sci. 2009; 32: 3070-83.
- Maurer HH. Perspectives of Liquid Chromatography Coupled to Low and High-Resolution Mass Spectrometry for Screening, Identification, and Quantification of Drugs in Clinical and Forensic Toxicology. Ther. Drug Monit. 2010; 32: 324-327.

