Ethylene Glycol
Ethylene glycol is a colorless, odorless, sweet-tasting, viscous liquid. It is primarily used as an automobile radiator antifreeze. Other uses include de-icing solutions, brake fluid, paints, lacquers, and as a solvent. Ethylene glycol intoxication is one of the most serious poisonings encountered in clinical toxicology. It is occasionally ingested as a beverage by a debilitated or misguided alcoholic patient and rarely by a child who has taken a sip of the sweet-tasting liquid. The lethal dose in adults is estimated to be 100 mLs. In children, the estimated lethal dose is 1mL per 1kg of body weight.
Chemistry, Absorption, and Metabolism
Ethylene glycol is a simple molecule. It is very similar to ethyl alcohol and its chemical structure is shown in (Fig. 1). Ethylene glycol is absorbed within 30 minutes of ingestion. Maximal blood concentrations are reached in 1 to 4 hours. The elimination half-life, in the absence of treatment, is 2.5 to 3.0 hours (1,2,3).
Figure 1. Structure of ethylene glycol

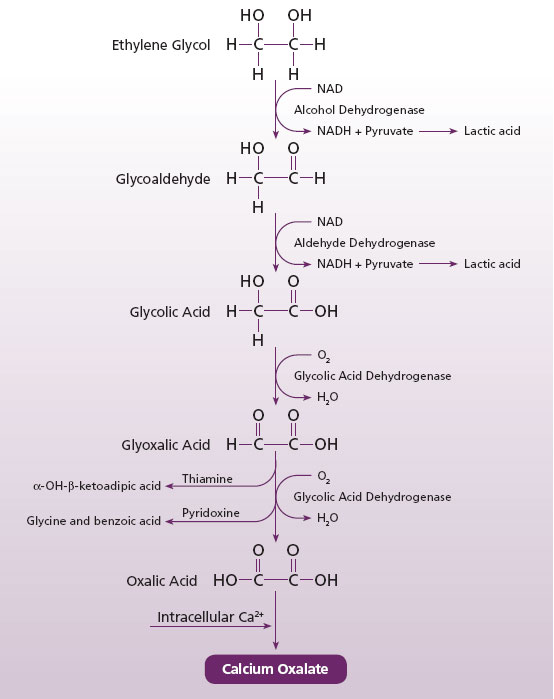
Unmetabolized ethylene glycol is not toxic. After ingestion, ethylene glycol is oxidized to glycoaldehyde, glycolic acid, glyoxalic acid, and oxalic acid (Fig 2). These four metabolites are responsible for the compound’s major toxic effects which include1 tissue destruction, primarily from calcium oxalate tissue deposition, and (2) metabolic abnormalities, specifically a high anion-gap metabolic acidosis, lactic acidosis, and hypocalcemia (4).
Figure 2. Metabolism of ethylene glycol
Clinical Presentation
Toxicity has been described as appearing in three stages (5,6).
Stage 1 occurs from 0.5 to 12.0 hours. CNS toxicity predominates with inebriation, confusion, nystagmus, paralysis, seizures, and coma. Nausea, vomiting, and papilledema may also occur. An elevated serum osmolarity is usually seen early in this phase. Calcium oxalate crystals may be present.
Stage 2 occurs from 12 to 24 hours postingestation. Cardiopulmonary symptoms predominate with mild tachycardia and hypertension. Other effects include anion gap metabolic acidosis (possibly severe) with compensatory hyperventilation, hypoxia, congestive heart failure, and acute renal distress syndrome.
Stage 3 occurs from 24 to 72 hours postingestion. This renal phase is characterized by flank pain with oliguria, hematuria, calcium oxaluria, proteinuria, and anemia leading to acute tubular necrosis and renal failure.
Ethylene Glycol Pharmacokinetic Data

Diagnostic Tests
Serum Ethylene Glycol Concentration
The determination of serum ethylene glycol concentration, usually by gas chromatography, is the preferred diagnostic procedure. If ethylene glycol levels are not available within a few hours, treatment should be initiated until the lab results become available. Levels greater than 20 mg/dL are considered toxic, but levels less than 20mg/dL may still indicate a toxic amount if significant time has passed since the ingestion.
Clinical Chemistry Tests
Serum osmolality may be useful if ethylene glycol levels cannot be done. The increased serum osmolarity is due to ethylene glycol and glycoaldehyde. The increase occurs early on and may disappear later as ethylene glycol and glycoaldehyde are metabolized. Thus the osmol gap may decrease despite a worsening toxicity. It should also be noted that an increase osmolality could be caused by other conditions such as shock, alcoholic or diabetic ketoacidosis, lactic acidosis or renal failure.
The presence of a high anion gap may be a useful diagnostic clue. The anion gap increases due partially to the formation of pyruvate to lactate in the first two steps of ethylene glycol metabolism7. However, the absence of an anion gap could be due to individual variability and does not rule out ethylene glycol ingestion.
Serum calcium testing and electrocardiogram monitoring are both indicated when monitoring an ethylene glycol poisoned patient.
Renal function tests and urinalysis should be done on symptomatic patients.
Calcium oxalate crystals may appear in the urine 4 to 8 hours after ingestion either as the monohydrate crystal (elongated) or the more specific dihydrate crystal (octahedral). These crystals will deposit in almost every tissue of the body including the brain, heart, lungs, kidneys, and urine.
Treatment
The treatment of ethylene glycol poisoning is complex and challenging. Gastric aspiration followed by lavage is useful up to one hour after ingestion. Bicarbonate drip is usually given not only to increase the bicarbonate concentration but also to promote the excretion of glycolic and lactic acids (7).
Antidotal therapy is indicated when 1) there is a history or suspicion of ethylene glycol ingestion 2) an unexplained increase in serum osmolarity 3) the presence of oxalate crystals in the urine or 4) ethylene glycol levels above 20 mg/dL (4).
Antidotal therapy is based on preventing the alcohol dehydrogenase enzyme from converting ethylene glycol into toxic metabolites (Fig 2). Two competitive inhibitors are currently in clinical use: ethanol and fomepizole. Ethanol has been the traditional antidote for ethylene glycol toxicity.
Ethanol has 100 times the affinity for alcohol dehydrogenase than ethylene glycol. In most cases, a serum ethanol level of 100 to 130 mg/dL is needed to effectively block alcohol dehydrogenase from metabolizing ethylene glycol. When ethanol is administered, serum ethanol concentrations should be monitored every one to two hours to avoid ethanol toxicity.
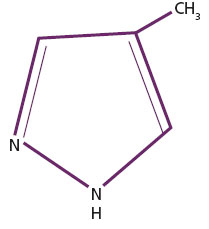
Fomepizole (Antizol) was approved by the FDA in 1997 for the treatment of ethylene glycol poisoning (Fig 3). It has 500 to 1,000 times the affinity for alcohol dehydrogenase than ethylene glycol. Fomepizole has advantages over ethanol in that it is easier to administer, causes no central nervous system depression, and has a longer duration of action. However the cost of the drug is much higher than ethanol (approximately $900.00 per dose).
Figure 3. Chemical structure of fomepizole (Antizol)
Thiamine is often given as an adjunct therapy (6). Besides the fact that many alcoholics are thiamine deficient, thiamine also prevents the formation of oxalic acid by facilitating the conversion of glycoxylic acid to alpha-hydroxy-beta ketoadipic acid (Fig 2).
Pyridoxine is also given as adjunct therapy. It prevents the formation of oxalic acid by converting glycoxylic acid to
benzoic acid and glycine (Fig 2).
Hemodialysis should be considered in the patient with severe metabolic acidosis, renal failure, or generally deteriorating condition. Traditonally, it has been recommended to dialyze the patient when the ethylene glycol level is greater than 50 mg/dL (8). However, if the patient is not acidotic, he or she may be treated with antidotal therapy only (9).
The endpoint of hemodialysis treatment or antidotal treatment should be a serum ethylene glycol level less than 20 mg/dL, with resolution of acidosis, and the patient should be improving clinically (10).
Summary
Ethylene glycol poisoning is a relatively rare event, but when it occurs it is a serious medical emergency. The measurement of ethylene glycol levels in serum and/or urine can confirm the diagnosis and may aid the clinician in both the type and duration of therapy.
References
- Peterson CD, Collins AJ, Himes JM, et al. Ethylene glycol poisoning: pharmacokinetics during therapy with ethanol and hemodialysis. N Engl J Med 1981;304:21-23
- Winek CL, Shingleton DP, Shanor SP. Ethylene and diethylene glycol toxicity. J Toxicol Clin Toxicol 1978; 13:297-324.
- Jacobsen D, Sebastian CS, Blomstrand R, et al. 4-Methylpyrazole: a controlled study of safety in healthy human subjects after single, ascending doses. Metab Clin Exp Res 1988; 12:516-522
- http://www.mnpoison.org/index.asp?pageID=155
- Berman LB, Schreiner GE, Feys J. The nephrotoxic lesions of ethylene glycol. Ann Intern Med 1957; 46:611-619.
- Kahn HS, Brotchner RJ. A recovery from ethylene glycol (antifreeze) intoxication; A case of survival and two fatalities from ethylene glycol including autopsy findings. Ann Intern Med 1950; 32:284-294.
- medicine.med.nyu.edu /... /Ethylene Glycol Poisoning.pdf
- Barceloux DG, Krenzelok EP, Olson K, Watson W. American Academy of Clinical Toxicology Practice Guidelines on the Treatment of Ethylene Glycol Poisoning. Ad Hoc Committee. J Toxicol Clin Toxicol 1999; 37:537-560.
- Borron SW, Megarbane B, Baud FJ. Fomepizole in treatment of uncomplicated ethylene glycol poisoning. Lancet 1999; 354:831.
- Heath A Joliff and Marco L.A. Swilotti. Ethylene Glycol. In Dart RC ed, Medical Toxicology, 3rd ed. Philadelphia Lippincott Williams and Wilkins 2004 p.

