Frequently Asked Questions about Human Papillomavirus (HPV) Testing
Papanicolaou (Pap) cervical cytology screening is one of the great success stories in modern medicine. Since its implementation in the 1950s, routine Pap screening has helped to reduce cervical cancer mortality by more than 70%.[1] Once the leading cause of cancer mortality in women, cervical cancer now ranks 13th in cancer deaths for women in the United States. [2] The very success of Pap screening has fostered an unrealistic expectation that Pap testing is perfect. In reality, the sensitivity of Pap cytology for high-grade cervical intraepithelial neoplasia (CIN) is only about 70 to 80 percent.[3] The relatively low sensitivity of Pap screening has stimulated a search for newer methods that could supplement or replace Pap testing. The leading candidate in this category appears to be nucleic acid testing for high risk human papillomavirus (HPV) types.
Human papillomaviruses (HPVs) are small, non-enveloped DNA viruses that replicate in the skin epithelium and mucus membranes of their host. More than 100 different HPV types (designated HPV type 1, HPV type 2, etc.) replicate in humans causing a wide range of symptoms. It is interesting to note that HPV symptomatology is often type-specific. Some HPV types (e.g., HPV types 6, 11, 42, 43, and 44) are more likely to produce warts while other types (e.g., HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68) produce malignancies of the skin and genital tract. The role of HPV in cervical cancer appears to be incontrovertible. Research in the past decade has shown that high-risk HPV types are present in 93 to 100% of all squamous cell carcinomas of the cervix.[4, 5]
Because of these observations, HPV testing is now one of the recommended strategies for managing patients with Pap results of “atypical squamous cells of undetermined significance” (ASCUS). [6] Managing patients with ASCUS is an important clinical challenge because only 5-10% of women with ASCUS have serious cervical disease. However, more than one-third of the high-grade squamous intraepithelial lesions (HSILs) in screening populations are identified from ASCUS Pap test results. [7]
HPV testing plus cytology has been found to be a promising approach for primary cervical cancer screening in women over the age of 30. [8] While the FDA has not yet approved HPV testing for this purpose, the new American Cancer Society Guidelines for the Early Detection of Cervical Cancer and Neoplasia reports that cervical screening of women over 30 years of age could be done every third year if the screening included a Pap test and an HPV test. [1] This proposed algorithm could not be used on younger women because most HPV infections in this age group are transient. [9,10]
A number of frequently asked questions are addressed below. For more information about HPV testing, contact Dr. Dan Wiedbrauk or Mary Lu Barth (800-876-6522).
How is HPV testing used in the management of patients with cervical abnormalities?
Of the estimated 50 million Pap smears performed each year in the United States, more than 5% are reported as abnormal. [11] There is a general consensus among health care providers that high-grade squamous intraepithelial lesions (HSILs) should be evaluated by colposcopy and biopsy. Until recently, however, there was no consensus regarding the management of women with low-grade squamous intraepithelial lesions (LSILs) or equivocal cytologic abnormalities (e.g., ASCUS).
The 2001 Consensus Guidelines for the Management of Women with Cervical Abnormalities published by the American Association for Colposcopy and Cervical Pathology state that a program of repeat cervical cytological testing, colposcopy, or DNA testing for HPV high-risk genotypes are all acceptable methods for managing women with a cytological diagnosis of ASCUS. [6]
Reflex testing for high-risk HPV genotypes is the preferred method when liquid-based cytology and/or cocollection for HPV DNA testing can be performed. [6] Under these guidelines, all women who test positive for HPV DNA should be referred for colposcopic evaluation. Women with ASCUS who test negative for HPV DNA can be followed up with repeat cytological testing at 12 months (Figure 1).
HPV-positive women who do not have biopsy-confirmed CIN may be managed with repeat cytological testing at 6 and 12 months and referral back to colposcopy if a result of ASCUS or greater is obtained. Alternatively, these women may be followed with repeat HPV DNA testing at 12 months with referral to colposcopy for all who are DNA-positive for HPV (Figure 1).
Can HPV testing be used to manage post-menopausal women with ASCUS?
Yes. Post-menopausal women with evidence of atrophy can be referred to immediate colposcopy or HPV DNA testing as shown in Figure 1. Women who have no contraindications to intravaginal estrogen therapy may be evaluated by repeat cytology one week after the completion of estrogen therapy. Patients with ASCUS or greater lesions on this cytological examination are referred to colposcopy. Patients with negative cytological results should be re-evaluated at 4-6 months. Patients with ASCUS or greater lesions on the third cytological exam are referred to colposcopy as before. Patients with repeatedly negative cytological testing can be returned to a normal screening schedule.
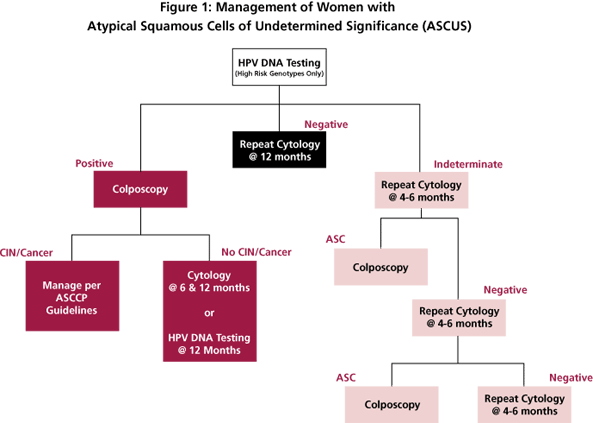
Algorithm for using HPV testing to manage women with atypical squamous cells of undetermined significance (ASCUS). Modified from reference 6.
There is no mention of an “Indeterminate” HPV result in the published studies. What does an “Indeterminate” HPV result mean?
Studies in our, and other, laboratories have shown that the Digene Hybrid Capture II HPV assay has low-end reproducibility issues. Specimens that initially test as very low-level positives may not be positive upon retesting. As a result, the laboratory cannot provide a definitive answer as to whether the specimen is truly positive or negative. Because HPV results are used to triage patients with cervical abnormalities, we felt that it is better to provide an indeterminate result rather than one that may not be reproducible. Patients with indeterminate high-risk HPV results can be managed via repeat cytology (Figure 1) or by colposcopy if the patient has had a previous ASCUS result.
What is the role of the low-risk probes in the management of women with cervical abnormalities?
Testing for low-risk HPV genotypes has little diagnostic value in the management of women with cervical abnormalities. The low-risk probe set (types 6, 11, 42, 43, and 44) tests for HPV genotypes that, by definition, are rarely associated with cervical cancer. Low-risk genotype HPV infections are more often associated with self-limited infections and benign genital warts.
Why did Warde Medical Laboratory reject liquid cytology specimens that are 3 weeks old?
The FDA-approved method for HPV testing states that ThinPrep™ specimens are stable for only three weeks. HPV DNA must be extracted within that timeframe in order for the testing to be valid. Our regulatory agencies require laboratories to validate any changes in the approved procedure. These validation studies are necessary to assure that the procedural modifications do not alter the sensitivity and specificity of the test procedure.
Warde Medical Laboratory recently tested a ThinPrep™ specimen that was more than three weeks old. Has there been a change in policy?
Yes, Warde recently completed a formal stability study on the ThinPrep specimens. Our validation data indicate that ThinPrep specimens are stable for at least 6 weeks at room temperature when stored in tightly sealed vials. Poorly sealed vials may have decreased stability due to alcohol evaporation.
Can you test other liquid cytology specimens for HPV?
Not at this time. The Cytyc ThinPrep™ product is the only liquid cytology system that has been approved by the FDA for testing using the Digene Hybrid Capture II HPV test. Warde Medical Laboratory has not performed the formal validation studies that would allow us to test other liquid cytology products for HPV. Alternate liquid cytology systems can be sent to a reference laboratory. Please contact Customer Services for the cost of this testing and for the specimen requirements.
HPV is a sexually transmitted disease. Why does Warde test only female specimens?
The Digene Hybrid Capture II assay is only approved for female cervical specimens and tissues. Research studies are ongoing at several institutions to examine the role of HPV in rectal cancers of gay men. However, testing of rectal mucosal specimens has not been approved by the FDA. Tissue specimens (condlylomas) from males may be tested in this assay. Tissue specimens must be fresh (unpreserved) and submitted to the laboratory in the Digene transport medium.
Literature Cited
- Saslow D, CD Runowicz, D Solomon, A-B Moscicki, RA Smith, HJ Eyre, and C Cohen. American cancer society guideline for the early detection of cervical neoplasia and cancer. CA 2002;52(6):342-362.
- The American Cancer Society, Cancer Facts & Figures, 2002.
- Sherman ME, M Schiffman, R Herrero, et al. Performance of a semiautomated Papanicolaou smear screening system: results of a population-based study conducted in Guanacaste, Costa Rica. Cancer 1998;84-273-280.
- Bosch FX, MM Manos, N Munoz, et al. Prevalence of human papillomavirus in cervical cancer: A worldwide perspective. International biological study on cervical cancer (IBSCC) study group. J Natl Cancer Inst 1995;87:796-802.
- Walboomers JMM, MV Jacobs, MM Manos, FX Bosch, JA Kummer, KV Shah, PJF Snijders, J Peto, CJLM Meijer, and N Munoz. Human papillomavirus is a necessary cause of invasive cervical cancer world-wide. J Pathol 1999;189:12-19.
- Wright, Jr. TC, JT Cox, LS Massad, LB Twiggs, and EJ Wilkinson. 2001 Consensus guidelines for the management of women with cervical cytological abnormalities. JAMA 287(16):2120-2129.
- Manos, MM, WK Kinney, LB Hurley, ME Sherman, J Shieh-Ngai, RJ Kurman, JE Ransley, BJ Fetterman, JS Hartinger, KM McIntosh, GF Pawlick, and RA Hiatt. Identifying women with cervical neoplasia using human papillomavirus DNA testing for equivocal Papanicolaou results. JAMA 1999;281(17);1605-1610.
- Kulasingham, SL, JP Hughes, NB Kiviat, C Mao, NS Weiss, JM Kuypers, and LA Koutsky. Evaluation of human papillomavirus testing in primary screening for cervical abnormalities: comparison of sensitivity specificity, and frequency of referral. JAMA 2002:288(14);1749-1757.
- Moscicki AB, S Shiboski, J Broering, et al. The natural history of human papillomavirus infection as measured by repeated DNA testing in adolescent and young women. J Pediatr 1998;132:277-284.
- Ho GY, R Bierman, L Beardsley, et al. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med 1998;338:423-428.
- Solomon D, M Schiffman, R. Tarone, and the ALTS Group. Comparison of three management strategies for patients with atypical squamous cells of undetermined significance: baseline results from a randomized trial. J Natl Cancer Inst 2001;93:293-299.

