Guidelines for the Clinical Use of ANA and Related Specific Autoantibody Testing
In January, 2000, the results of a National Consensus Conference on Guidelines for ANA testing was published (Kavanaugh et al.). The panel of scientists included representatives from the Clinical Center of the National Institutes of Health, the Clinical Immunology Society, the American College of Rheumatology, and the College of American Pathologists. This article is based upon the findings of that consensus group.
Because almost all (98-99%) patients with systemic lupus erythematosus have anti-nuclear antibodies (ANA) demonstrable in their serum, testing for ANA can be very useful. It is, however, far from a perfect test. ANA may be found in a variety of conditions and in normal individuals. Therefore, careful selection of patients to be tested is of great importance. Improper use of ANA testing can lead to misinformation, incorrect diagnoses, and a waste of limited revenue for testing.
History of ANA Testing
The first test for detecting systemic lupus erythematosus (SLE) was the “LE cell” test that was developed in 1948 by Hargraves. It consisted of taking peripheral blood, damaging the cells (one method used glass beads), and then incubating the sample. Those samples that had antibodies to nuclei would react with the disrupted lymphocyte nuclei and opsonize them to be phagocytosed by neutrophils in the blood. The samples were then stained with a Wright stain and examined by a technologist. This test was not very sensitive, being positive in only about 50% of patients with SLE. Yet, it was the only test available in the late 1940’s. This test should no longer be used because it is insensitive and the interpretation is subjective.
| History of ANA sensitivity for SLE |
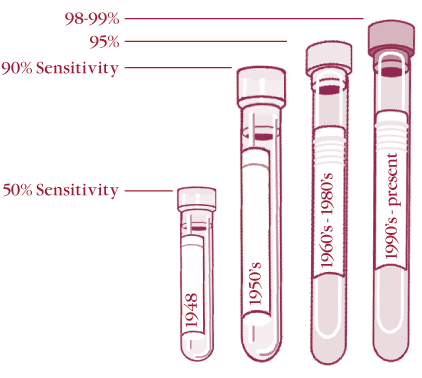 |
By the late 1950’s, it was discovered that a high percentage of patients with SLE had circulating antibodies that could be detected by an immunofluorescence test on frozen sections of rodent kidney or liver. While these would pick up about 90% of patients with SLE, they would miss patients who had weaker titers of anti-nuclear antibodies, as well as most of the patients who had anti-SSA/Ro as their predominant antibody. By changing the substrate to a tissue culture line in the late 1960’s and improving fixation in the 1980’s, we can now detect 98-99% of patients with SLE. Unfortunately, the new techniques also pick up small amounts of ANA that occur in controls. To remedy this, manufacturers of ANA kits have increased the screening dilutions from the 1:20 or 1:40 used on the older (less sensitive) tissue section substrates, to 1:80 or even higher.
The Guidelines panel reported that with the 1:40 screening dilution as many as 20-30% of serum from normal individuals would give a positive result. Using a 1:80 screening dilution, false positive rates dropped to about 10% and at 1:160 it was down to about 5%. Some laboratories today still screen at 1:40 which will give a high rate of false positivity.
The newest screening technique for ANA is the enzyme immunoassay (EIA). The EIA uses nuclear antigens that have been purified from cell preparations or antigens that are prepared by recombinant methods. These antigens are placed in a microtiter well for testing. In our laboratory, we have found that this method provides fewer false positives than with the tissue culture cells, while retaining the high sensitivity for detecting SLE.
Conditions where ANA testing provides information that is very or somewhat useful for establishing the diagnosis
The Guidelines panel reviewed the medical literature and their own clinical expertise to define the conditions where ANA testing was useful for Diagnosis (Table 1). Only two diseases were categorized as having an ANA test provide information that is very useful for diagnosis-SLE and systemic sclerosis (scleroderma).
| Table 1 Diseases where ANA testing is very or somewhat useful for diagnosis | |
| DISEASE | PERCENT ANA POSITIVE |
| SLE | 95-100 |
| Scleroderma | 60-80 |
| Sjögren’s Syndrome | 40-70 |
| Dermatomyositis or Polymyositis | 30-80 |
| From Kavanaugh et al. Arch Pathol Lab Med 2000;124:71 |
| Table 2 Criteria for the classification of Lupus Erythematosus | |
| Malar rash | |
| Discoid rash | |
| Photosensitivity | |
| Oral ulcers | |
| Arthritis | |
| Serositis | |
| Renal disorder | |
| Neurologic disorder Seizures Psychosis | |
| Hematologic disorder Hemolytic anemia Leukopenia Thrombocytopenia | |
| Immunologic disorder Anti-DNA Anti-Sm False positive test for syphilis | |
| Antinuclear antibody | |
| Modified from Keren and Hedstrom, J. Clin ligand Assay 1999; 22:50. | |
An ANA test is needed if there is a reasonable suspicion of SLE from either history, physical findings or the results of routine tests such as the CBC (Table 2). Currently available EIA and Immunfluorescence tests are highly sensitive for detecting patients with SLE. Because there are only about 50 cases of SLE per 100,000 individuals in a population, the random chance of a false positive in normal individuals is considerably greater than a true positive, unless the clinician selects only individuals with a reasonably high possibility of having the disease before testing. This is because, even with the newest tests, and greater screening dilutions (1:160) about 5% (1 out of 20) of normal individuals will give a weakly positive result. If one tested all normal individuals, then, in 100,000 controls one would find 5,000 positive results. Clearly, this number dwarfs the 50 true cases of SLE that would be in a population of 100,000 people. This is why pretest selection of patients with appropriate symptoms is so important (Table 2). ANA should never be ordered as a routine screening test.
The Guidelines panel was emphatic that a positive ANA result in a patient with no or minimal features of SLE can be misleading or worse because it may cause the clinician to order unnecessary tests. An old aphorism is that “bad information is worse than none”. The panel cautioned that a positive ANA in individuals lacking appropriate symptoms of SLE may lead to an erroneous diagnosis or inappropriate therapy. The ANA should only be used as supportive evidence of this disease.
The other condition where ANA testing may be very useful for diagnosis is scleroderma. Although a positive ANA is not required for the diagnosis of scleroderma, as shown in Table 1, 60-80% of these patients have a positive ANA. A positive ANA provides supportive information for those individuals who have the protean signs and symptoms associated with scleroderma.
Although not as useful as in SLE or scleroderma, a test for ANA is helpful in patients with Sjögren’s syndrome and in those with dermatomyositis or polymyositis. In Sjögren’s syndrome, typically more than half of the patients will have a positive ANA and it can provide supportive information in individuals who have sicca syndrome. The most common specific antibodies found in Sjögren’s syndrome are anti-SSA-Ro and SSB-La. The former is also found in neonatal lupus.
Dermatomyositis and polymyositis are idiopathic inflammatory conditions of muscle where the ANA (typically found in about half of the patients) may provide supportive information. Clearly, with only half of the patients in this category being positive, a negative test does not rule out the disease. Appropriate diagnostic procedures, such as biopsy, are needed to establish the diagnosis.
There are a few conditions where the medical literature requires the presence of a positive ANA for the diagnosis. Whether this really reflects a specific medical condition, or is an artificial criterion that was part of early studies on these entities, the requirement for ANA testing has remained. These conditions include drug-induced lupus, autoimmune hepatitis and mixed connective tissue disease.
Conditions where the ANA test is not useful information for diagnosis
The Guidelines panel listed a number of conditions where the ANA test is positive more often than in the general population, but where it is present in too low a proportion of the affected individuals to be of diagnostic help. Those conditions are listed in (Table 3).
| Table 3 Do not order ANA on these diseases where ANA testing is not useful for diagnosis | |
| DISEASE | % ANA POSITIVE |
| Rheumatoid Arthritis | 30 |
| Multiple Sclerosis | 25 |
| Discoid Lupus | 5-25 |
| Idiopathic Thrombocytopenic Purpura | 10-30 |
| Patients with Silicone Implants | Same as controls |
| Fibromyalgia | 15-25 |
| From Kavanaugh et al. Arch Pathol Lab Med 2000;124:71 |
Other tests are available for some of these conditions which provide much more useful information. For instance, rheumatoid factor assay that is available by nephelometric or agglutination techniques can be helpful in the diagnosis of rheumatoid arthritis. CSF oligoclonal bands and IgG index are useful tests in supporting the diagnosis of multiple sclerosis.
Discoid lupus lesions are erythematous, hyperkeratotic scarring cutaneous lesions often with an overlying scale. The ANA test is not useful for this diagnosis. The history and physical examination will determine whether further testing for systemic disease is worthwhile. About 5-10% of women with discoid lupus on presentation will progress to systemic disease. Conversely, about 15% of patients with SLE also have discoid lesions. Patients with idiopathic thrombocytopenia purpura have antiplatelet antibodies. The presence or absence of ANA is not useful in distinguishing cutaneous discoid and systemic lupus.
Women with silicone breast implants are at no increased risk for developing either defined or vague autoimmune disease. ANA and other autoimmune tests on asymptomatic women with silicone implants are a waste of time and limited testing resources. Indeed, since 10% or more of controls may give positive results, depending on the screening dilution used (see above), a positive result provides misleading information. Of course, if there are clinical symptoms of SLE in such patients, they should receive the routine testing that would be performed in that situation.
There is no useful autoimmune test for fibromyalgia. It is a clinical diagnosis where the American College of Rheumatology has established clinical criteria that are useful in the diagnosis (Wolfe F, et al).
The panel also recommended that asymptomatic relatives of patients with autommune diseases not be tested for ANA reactivity.
Conditions where ANA testing provides information that is useful for monitoring the disease or for prognosis
Juvenile chronic arthritis (JCA) was formerly termed juvenile rheumatoid arthritis. However, since it is a different disease from the adult rheumatoid arthritis and is usually negative for rheumatoid factor, in recent years the term JCA has been used. Although the ANA test is not useful in establishing the diagnosis of JCA, it may help to identify those children with JCA who are more likely to develop uveitis. About 30% of the JCA patients who are ANA positive will develop uveitis and would best benefit from more detailed ophthalmologic examination.
Raynaud phenomenon is also not diagnosed by performing an ANA. It is identfied by the specific complaints elicited by the patient’s history and the physical findings. As with JCA, once the diagnosis is established clinically, a positive ANA test increases the likelihood that a patient will develop a specific systemic rheumatic disease from about 19% to 30%. A negative ANA decreases the likelihood from 19% to 7%. Therefore, a positive result necessitates following up for the development of systemic autoimmune disease.
Antiphospholipid antibody syndrome (APS) is diagnosed by a combination of clinical features of skin rash and/or ulcers, increased arterial and venous thromboses, recurrent fetal loss, and thrombocytopenia. Specific tests for anti-cardiolipin antibodies (specifically beta-2 glycoprotein I) and for lupus anticoagulant are available (Triplett, Warde Report 2000). The ANA test is not needed for the initial diagnosis of APS. However, once the diagnosis is established, the presence of a positive ANA increases the chances that the APS is related to the presence of SLE.
An ANA may be useful for prognosis in only these 3 conditions. It was further noted that the presence of an ANA even in diseases such as SLE, where it is useful for diagnosis, does not provide useful information for prognosis or monitoring. Therefore, once the diagnosis of SLE is established, there is no need to subsequently monitor the ANA level.
Other Testing as a Follow-up to a Positive ANA Test Result
When the ANA is positive, it confirms that some antigen within the nucleus of the cell reacts with the patient’s serum. However, there is a considerable diagnostic difference when that something is Scl-70 (topoisomerase 1) that is relatively specific for progressive systemic sclerosis (scleroderma) versus Sm that is highly specific for SLE. There are many other specific tests available including anti-dsDNA, anti-Sm, anti-nRNP, anti-Ro (SS-A), anti-La (SS-B), anti-centromere, anti-Scl-70, and anti-Jo-1 (Table 4).
Although some laboratories offer large batteries of tests for specific ANA antigens, this type of shotgun testing is to be deplored. The laboratories stand to profit from the many tests. As a justification for these useless, costly panels, they offer the unsubstantiated “security” that nothing will be missed. They don’t deal with the issue raised by the panel that this type of shotgun testing results in increased costs and leads to erroneous diagnoses due to false positives and false negatives. These tests should be used judiciously.
The panel suggested using the clinical differential diagnosis to guide testing (Table 4). For instance, when one suspects SLE by the criteria in Table 2 above, tests for anti-dsDNA and anti-Sm are useful. Anti-ssDNA is not needed (ever). It is not specific and its absence does not exclude anything. Even anti-dsDNA is not entirely specific. It can be detected in serum from patients who suffer from other conditions. dsDNA is not present in all patients with SLE. Approximately 50% of patients with SLE will have demonstrable anti-dsDNA. There is also variation in detection of anti-dsDNA depending on the method utilized. Newer enzyme immunoassay methods for dsDNA are more sensitive than the older Crithidea luciliae indirect immunofluroescent method, but the latter may be more specific.
| Table 4 Guide to follow-up testing when the ANA is positive | |
| CLINICAL SUSPICION | FURTHER SPECIFIC TESTING |
| SLE | Anti-dsDNA, Anti-Sm |
| Neonatal Lupus | Anti-Ro (SS-A) |
| Scleroderma | Anti-Scl-70, Anti-Centromere* |
| Sjögren’s Syndrome | Anti-Ro (SS-A), Anti-La (SS-B) |
| Polymyositis or Dermatomyositis | Anti-Jo |
| Drug-Induced Lupus | No further testing |
| Mixed Connective Tissue Disease | Anti-nRNP |
| *Anti-Centromere is included when immunofluorescence is used for ANA screening. It may need to be added if enzyme-immunoassay is the screening test. |
Some of the antibodies in patients with SLE are formed against nuclear RNA proteins. Antibodies against Sm (Smith) is an anti-ribonucleoprotein antibody that is highly specific for SLE, but is only present in about 20% of patients with SLE. In contrast, anti-nRNP that form to other ribonucleoproteins are present in about 35% of SLE patients, but is not recommended for follow-up testing when SLE is suspected because it is not specific for SLE and cannot rule out other conditions (such as mixed connective tissue disease-MCTD).
Other specific antibodies are noted in Table 4. Of interest, there is no further testing warranted in drug-induced lupus. The presence of a positive ANA is part of the diagnostic criteria for the condition. Once the drug is discontinued, the clinical condition should improve quickly. The positive ANA may persist for a year or longer, so following the titer or presence of ANA is of no use. Tests for antibodies to histones are not needed for the diagnosis and are useful only in a research setting.
Tests that are useful for Prognosis in ANA Positive Samples
There is a weak correlation between the presence of anti-dsDNA and the development of renal involvement and its severity. However, the presence of anti-dsDNA certainly does not mean the patient will develop renal disease, and its absence does not exclude lupus nephritis. This is something that must be followed with clinical evaluation. The higher the titer of anti-dsDNA, the more likely it is that there is active disease.
Anti-Ro (SS-A) is associated with neonatal lupus that may cause rash and complete congenital heart block. The panel suggested screening for anti-Ro (SS-A) in pregnant women who have SLE to monitor for this complication. Anti-Ro (SS-A) is also associated with photosensitivity, Sjögren’s syndrome (as mentioned above), thrombocytopenia and subacute cutaneous lupus erythematosus.
For patients with scleroderma, the presence of anti-centromere antibodies increases the likelihood that the form of disease will be the relatively limited CREST syndrome: calcinosis, Raynaud phenomenon, esophageal dysmotility, sclerodactyly and telangiectasias.
Further Reading
Kavanaugh A, et al. Guidelines for clinical use of the antinuclear antibody test and tests for specific autoantibodies to nuclear antigens. Arch Pathol Lab Med 124:71,2000. Keren DF, Hedstrom DL. Contemporary approaches to anti-nuclear antibody serology. J Clin Ligand Assay 22:50, 1999. Triplett, D. Anticardiolipin antibodies (ACA): Antigenic targets and pathophysiology. Warde Report 11:1, 2000. Wolfe F et al. The American College of Rheumatology criteria for the classification of fibromyalgia: report of the Multicenter Criteria Committee. Arch Rheum 33:160, 1990. TopContinuing Education Quiz
| The LE cell test: | |||
| A | is performed routinely at most hospital laboratories | ||
| B | is considered the best diagnostic test for systemic lupus erythematosus | ||
| C | is an objective measure of anti-dsDNA antibodies | ||
| D | has been replaced by more specific tests | ||
| E | demonstrates an eosinophil engulfed by a macrophage | ||
| Answer | |||
| The ANA test is very or somewhat useful for diagnosis of all but one of the following (pick the one which is not recommended). | |||
| A | Dermatomyositis | ||
| B | Sjögren's syndrome | ||
| C | Fibromyalgia | ||
| D | Systemic lupus erythematosus | ||
| E | Scleroderma | ||
| Answer | |||
| Panels of tests that include anti-dsDNA, -Sm, -nRNP, -centromere, -Scl-70, -Ro, -La, and -Jo-1: | |||
| A | offer a streamlined way of working up a patient with vague autoimmune symptoms | ||
| B | are highly cost efficient | ||
| C | are not recommended because of misinformation and high cost | ||
| D | are best performed during periods of disease remission | ||
| E | are exquisitely specific for SLE | ||
| Answer | |||
| EXTRA CREDIT Groucho Marx got his name from: | |||
| A | the name given to him as a baby | ||
| B | it was a nickname from childhood when he was grouchy | ||
| C | it was derived from a character in the Sherlocko comic strip | ||
| D | his mother's family was Italian and this was his uncle's name | ||
| E | the New York Time's review of the Broadway production of "Animal Crackers" first used this to describe him on stage | ||
| Answer | |||

