HIV Screening by BioPlex
This summer, Warde Medical Laboratory will transition to a new method for initial screening for infection with human immunodeficiency virus (HIV). Currently, the Laboratory uses a 4th generation HIV screen– so-called because, in addition to assessing for the presence of antibodies to HIV-1 and HIV-2 (as was the case for 3rd generation screens), it also assesses for the presence of the HIV-1 p24 antigen, enabling initial detection in the early phases of infection, potentially weeks before the development of anti-HIV antibodies. The new platform, sometimes referred to as “5th generation,” 1 also assesses for p24 antigen in addition to HIV-1 and HIV-2 antibodies, but allows for the separate determination of each of these components in a single reaction vessel.
The new method is manufactured by BioRad under the BioPlex brand, and is based in Luminex bead technology. This method uses microscopic beads coated with different ligands specific to the detection of each particular analyte. This allows for separate detection of antibodies to HIV-1 (groups M and O), antibodies to HIV-2, and detection of the HIV-1 p24 antigen at the time of initial screen (see Figure). In a process analogous to flow cytometry, beads are passed through a pair of lasers after initial incubation, where bound antibody (or bound antigen) is detected using a fluorochrome tag.
Since the current CDC algorithm for diagnosis of HIV infection still requires a supplemental method for initial reactive screens, we will still be following up initial antibody-positives with the BioRad Geenius HIV 1/2 differentiation assay. As such, users likely will not see major changes in test resulting and formatting.
Overall, this multiplex approach to HIV screening has similar sensitivity and specificity to existing 4th generation platforms,2, 3 but offers some advantages over previous methods. Some users may see an improvement in turnaround time due to greater random access capability for required repeats of initial positives, and due to the potential for more rapid auto-release of negative results. Also, the separate identification of “antigen-only” positives might abrogate the need for supplemental antibody testing in some cases.
Initially, the BioPlex will be used only for HIV screening, but our acquisition of this platform could allow for the adaptation of many different immunoassay-based serologic studies, potentially increasing efficiency in laboratory operations beyond HIV screening.
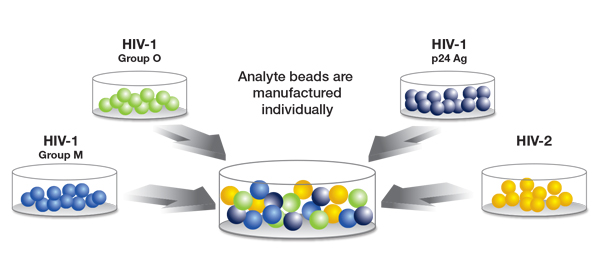
Figure courtesy of BioRad
- Salmona M, Delarue S, Delauigerre C, Simon F, Maylin S: Clinical evaluation of BioPlex 2200 HIV Ag-Ab, an automated screening method providing discrete detection of HIV-1 p24 antigen, HIV-1 antibody, and HIV-2 antibody. J Clin Microbiol 2014; 52(1):103-107.
- Eshleman SH, Piwowar-Manning E, Sivay MV, Debevec B, et al: Performance of the BioPlex 2200 HIV Ag-Ab assay for identifying acute HIV infection. J Clin Virol 2018; 99-100:67-70.
- Qiu X, Sokoll L, Yip P, Elliott DJ, et al: Comparative evaluation of three FDA-approved HIV Ag/Ab combination tests using a genetically diverse HIV panel and diagnostic specimens. J Clin Virol 2017; 92:62-68.

