Laboratory Evaluation for Monoclonal Gammopathies: MGUS to Myeloma
Since 1847 when Henry Bence Jones was referred to as “the best chemical doctor in London” by Florence Nightingale, we have had a quantifiable tumor marker, monoclonal free light chains (mFLC) to aid in our evaluation of patients with multiple myeloma (MM). Since MM is responsible for about 10% of hematological malignancies, it is a disease one wishes to rule out when individuals in their sixth decade and beyond present with bone pain. The acronym CRAB (elevated Calcium, Renal insufficiency, Anemia, and Bone lesions) is a convenient way to remember the key presenting features of MM.
Conditions with M Proteins
Whereas the detection and measurement of M proteins are of great importance in evaluating patients who have
symptoms of malignant hematologic conditions, MM is just one of several plasma cell proliferative disorders that will be
snared by the same techniques we use to detect MM (Table 1).
Table 1. Conditions with M Proteins
| Conditions | Clinical Effect |
| Multiple Myeloma | Severe |
| Waldenström’s Macroglobulinemia | Moderate |
| AL Amyloid | Severe |
| Smoldering Multiple Myeloma (SMM) | Asymptomatic with risk of progression |
| Monoclonal Gammopathy of Undetermined Significance (MGUS) | Asymptomatic with risk of progression |
| Transient monoclonal | Usually reactive (infection, occasionally autoimmune) |
| M Protein-Associated Neuropathy | Moderate to Severe |
| Plasmacytoma | None to moderate |
Unfortunately, since the vast majority of M proteins detected by serum protein electrophoresis are caused by a nontreatable condition, monoclonal gammopathy of undetermined significance (MGUS), a screening process that spreads its net too broadly will be both expensive and counterproductive. In this article, I review strategies for efficient use of currently available techniques for detecting M proteins to provide a cost-effective means for detection of the clinically significant treatable conditions among this group of diseases.
MGUS is clinically asymptomatic and progresses to MM at the rate of only 1% per year. Considering that most cases of MGUS are detected in their seventh decade or later, it does not have much prognostic significance for many of those patients. Patients with smoldering multiple myeloma (SMM) like those with MGUS are asymptomatic, but annually have a 10% chance of progressing to MM or AL Amyloidosis. For the latter individuals, a more aggressive follow-up is prudent.
Because they are clinically asymptomatic, MGUS and SMM are detected by the chance discovery of an M protein when patients with vague symptoms, such as nausea and fatigue are screened by serum protein electrophoresis. Once detected, however, one is obliged to further evaluate them because the risk of either condition progressing to MM can be stratified by quantifying readily available risk factors. For patients with MGUS, three factors can determine the risk of progression (Figure 1).
Figure 1. Progression of MGUS to MM over 20 years. (Data from Rajkumar et al 2005)
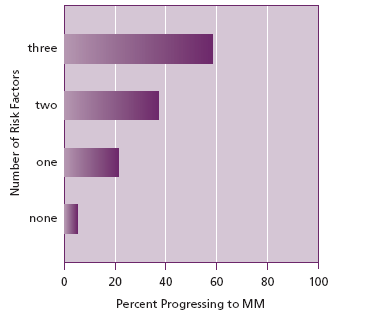 First, IgA and IgM M proteins are more likely to progress than IgG M proteins. Second, M proteins greater than 1.5 g/dl are more likely to progress than those of lesser quantity. And third, M proteins with abnormal serum free κ/λ ratios (implying the presence of a monoclonal free light chain, ie Bence Jones protein) are also a greater hazard for the patient. If all 3 risk factors are present, the absolute risk of progression to MM or a B cell lymphoproliferative neoplasm at 20 years is 58%, but if no factor is present the risk of progress is only 5%. Determining this difference allows appropriate frequency of monitoring depending upon risk.
First, IgA and IgM M proteins are more likely to progress than IgG M proteins. Second, M proteins greater than 1.5 g/dl are more likely to progress than those of lesser quantity. And third, M proteins with abnormal serum free κ/λ ratios (implying the presence of a monoclonal free light chain, ie Bence Jones protein) are also a greater hazard for the patient. If all 3 risk factors are present, the absolute risk of progression to MM or a B cell lymphoproliferative neoplasm at 20 years is 58%, but if no factor is present the risk of progress is only 5%. Determining this difference allows appropriate frequency of monitoring depending upon risk.
Laboratory Tests to Evaluate Patients with M Proteins
When evaluating patients for M protein-related hematologic malignancies, screening optimally includes a complete blood count with a review of the peripheral smear, routine chemistries with calcium, creatinine, albumin, total protein, as well as quantification of IgG, IgA and IgM. In cases of MM, one or more of these parameters typically are abnormal suggesting that further testing for an M protein would be fruitful.
The M protein immunoglobulin product and/or its monoclonal free light chain (mFLC) counterpart are the distinguishing features in all cases of MM, except the 1-2% nonsecretory ones. There are five main tests that clinical laboratories use for detection of M proteins.
Figure 2. Normal SPE on left. SPE with an M protein on the right.
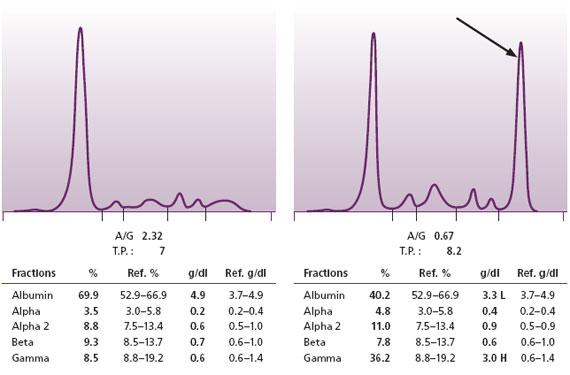
Serum Protein Electrophoresis
The mainstay of these five techniques is serum protein electrophoresis (SPE) (Figure 2). The SPE is a handy test because it demonstrates all the major protein fractions of serum: albumin and the globulins (alpha1, alpha2, beta, and gamma). With this procedure, one can readily detect large quantities of intact M proteins, hence most of the cases of MM and Waldenström’s. Further, a well-trained interpreter can also pick up more subtle changes that indicate the presence of small quantities of M proteins.
Not all malignant plasma cell clones produce the vast quantities of M protein found in most cases of MM. In some cases, only scant quantities of M protein are produced, and in a very few cases no M protein is detected at all. At Warde Medical Laboratory, we measure all detected M proteins down to the level of 0.1 g/dl. When we report even such small quantities of M proteins in the serum we urge the clinician to follow-up with a repeat sample as well as to check for the presence of monocolonal free light chains. This is because even a puny M protein barely bobbing above the surface of background immunoglobulins may herald a huge mFLC.
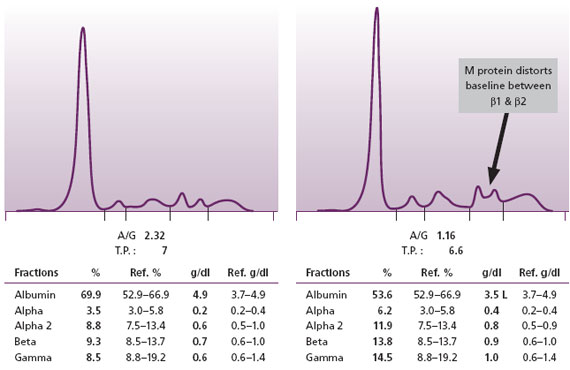
When an intact M protein in MM is produced in relatively small amounts, its presence may be obscured because it co-migrates with prominent beta or alpha region proteins. Narayan et al. (2003) have suggested that distortions of beta region bands or the baseline without an obvious explanation of an alternative etiology, such as a liver disease pattern, may indicate the presence of a small quantity of M protein (Figure 3). However, some intact M proteins are present in such small quantities that they cannot be detected even by sensitive high resolution serum protein electrophoresis techniques. This is why we consider that SPE alone is not a sufficient screen for the MM.
Figure 3. Normal SPE on left. On the right is an SPE with a small M protein that has resulted in an increased baseline (a bridge) between the two normal beta region bands. At Warde Medical Laboratory, such patterns are reflexed to serum immunofixation (IFE) to demonstrate the M proteins.
Serum Free Light Chain Test
The second most important screening technique in use today is the serum FLC assay. This test has revolutionized the detection of mFLC. It has been used for almost 10 years now and the detection and measurement of serum FLC has improved several problematic areas in evaluating patients for MM. The serum FLC assay can replace the need to send urine along with serum for the initial evaluation of patients suspected of having MM. The older standard was to perform urine protein electrophoresis and immunofixation on either an early morning void or a 24 hour urine collection. Unfortunately, because such samples were often not forthcoming, diagnosis of cases with light chain only or light chain predominant MM occasionally was delayed. Katzmann et al demonstrated that 99.5% of cases of MM with mFLC could be demonstrated by using serum FLC methods along with SPE and serum IFE to replace the urine sample.
Beyond improving the initial detection of more typical cases of light chain predominant MM, the serum FLC test detects mFLC in more than half of the 1-2% of MM cases previously referred to as “nonsecretory” myeloma. The term “oligosecretory” is now applied to such cases where an abnormal serum FLC K/L can be demonstrated. The presence of this mFLC in such cases has made it easier to follow therapy in those cases, since bone marrow biopsies formerly were required to provide the clinician with an objective assessment of the tumor burden. In addition to oligosecretory myeloma, an abnormal FLC is present in more than 90% of cases of AL Amyloidosis, the most common form of amyloidosis. Once again, the quantitative nature of this straightforward and reproducible test has been used to effectively monitor the response to therapy. Further, as noted above, serum FLC is used to help stratify the risk of patients with MGUS and SMM. Because of these findings, Warde Medical Laboratory recommends the combined use of SPE and serum FLC on all patients for their initial evaluation for MM or Waldenström’s macroglobulinemia.
Serum Immunofixation
The third commonly used technique to detect M proteins is serum immunofixation (IFE). At Warde Medical Laboratory, on the initial evaluation of all samples with an M protein demonstrated by SPE, we recommend performing an immunofixation to identify the heavy and light chain type. This information is necessary for determining the risk of progression of MGUS and SMM cases, and has prognostic implications for individuals with MM. IFE is also highly recommended in individuals who have M proteins associated with peripheral neuropathies. IFE must be ordered on all cases where a monoclonal myelin-associated anti-glycoprotein antibody is suspected of causing a peripheral neuropathy because they are frequently present in relatively small quantities that may be missed by SPE alone. Latov et al. demonstrated that SPE alone is too insensitive to detect the (usually) IgM M proteins found in patients with myelinassociated anti-glycoprotein monoclonal antibodies. Their findings are supported by the recent Katzmann et al. report (see below) in which a quarter of the cases of M proteins in patients with POEMS (peripheral neuropathy, organomegaly, endocrine disorders, M protein, and skin changes) syndrome were too small to be detected without IFE.
Urine Protein Electrophoresis
The fourth commonly used technique to screen for M proteins is urine protein electrophoresis (UPE). This test requires either an early morning void (before the patient had swallowed several cups of liquid) or a 24 hour urine specimen. The sample must be concentrated at least 50X and electrophoresis is performed separating the same fractions as by serum protein electrophoresis. This method detects specific patterns of proteinuria (glomerular, nonselective, tubular, and overflow) that can be helpful, but are not critical to the initial diagnosis of the malignant hematologic conditions associated with M proteins. Because deposition of mFLC can be present in AL Amyloidosis or serum free light chain disease, the finding of specific patterns of proteinuria may encourage further evaluation for those conditions. Large quantities of mFLC can be detected by UPE, however relatively small quantities may be missed by this technique which is why it adds relatively little to the initial screen. Once it has been established that an mFLC is present in the urine, measurement of this on a 24 hour urine sample has been valuable for following patients who have, predominantly or exclusively, mFLC as the product of the malignant plasma cell clone. However, the serum FLC assay has an advantage of measuring quantities of FLC that may be too small to see even by urine IFE and may eventually replace the 24 hour urine test.
Urine Immunofixation
The fifth commonly used technique is urine IFE. Until recently, this test was highly recommended for the initial evaluation of patients suspected of having MM since in about 15% of cases of MM only a mFLC is present with no, or trivial amounts of the intact immunoglobulin M protein. However, because the combination of SPEP and serum FLC is highly effective as a screening strategy for detecting the cases of MM and Waldenström’s macroglobulinemia, the need for urine IFE is now more related to the detection of AL Amyloidosis in the few cases that have a normal serum FLC, or to distinguish the mFLC from the intact immunoglobulin M proteins in cases where urine monitoring of FLC is needed for following therapy in some research protocols.
Efficacy of the 5 Tests for Detecting Clinically Significant Conditions
Better perspective on the capabilities of these five tests for M proteins was provided by a Katzmann et al.’s landmark analysis of 1877 patients classified in the dysproteinemia database of all Mayo Clinic patients with an M protein who had had all five M protein assays performed (Table 2). This study found that all 467 cases of active MM and all 26 cases of Waldenström’s macroglobulinemia were detected by using just the SPE together with serum FLC tests. For SMM, the SPE with serum FLC test together detected 190 cases, by adding serum IFE only one additional case was found. There is no current recommendation to screen for cases of SMM and since the serum FLC test for this case was negative, and its quantity was too small to detect by SPE alone, it would be unlikely that this case of SMM would have been in the higher risk groups with regard to progression to MM.
A few other treatable M protein-related conditions were also studied by Katzmann et al. Of the 581 cases of AL Amyloidosis, the use of all five tests detected 98% of cases while the use just SPE together with FLC detected 96%. The 2% that could be detected required either urine or serum IFE as additional screening tests. However, even with all 5 tests, 11 cases of AL Amyloidosis required biopsy confirmation. For LCDD, of the 18 cases in the study, 15 were detected by the use of all five tests, 14 by PEP and FLC. Therefore, if either AL Amyloidosis or LCDD are in the differential, I recommend that if the initial screen with SPE and serum FLC is negative, an immunofixation of both serum and urine and biopsy of affected organs be performed.
Of the 29 cases of plasmacytoma, in Katzman et al’s series, 25 were detected by PEP with FLC with only one additional case picked up by performing all five tests. However, only 2 of the 10 cases of extramedullary plasmacytomas were detected even by using by all five tests. If extramedullary plasmacytoma is part of the differential, imaging and biopsy will be needed to rule out negative screening studies by these five methods.
As mentioned above, the cases of M protein-associated neuropathies missed by SPE and FLC were all detected by serum IFE. Because of this, Warde Medical Laboratory recommends that all patients with peripheral neuropathies that may be related to M proteins have a serum IFE ordered. For these cases urine IFE and FLC are not required.
If no M protein is detected by all five methods, but active MM is still suspected by the clinical symptoms a bone marrow biopsy needs to be performed to detect cases of nonsecretory MM.
Table 2. Clinical Study: Detection of M proteins†
| Condition (n) | All 5 tests* | SPE/FLC/IFE | SPE & FLC | Serum IFE |
| MM (467) | 100 (467)** | 100 (467) | 100 (467) | 94.4 (441) |
| Waldenström’s (26) | 100 (26) | 100 (26) | 100 (26) | 100 (26) |
| SMM (191) | 100 (191) | 100 (191) | 99.5 (190) | 98.4 (188) |
| MGUS (524) | 100 (524) | 97.1 (509) | 88.7 (465) | 92.8 (486) |
| Plasmacytoma (29) | 89.7 (26) | 89.7 (26) | 86.2 (25) | 72.4 (21) |
| POEMS (31) | 96.8 (30) | 96.8 (30) | 74.2 (23) | 96.8 (30) |
| Extramedullary plasmacytoma (10) | 20 (2) | 10 (1) | 10 (1) | 10 (1) |
| Primary AL (581) | 98.1 (570) | 97.1 (564) | 96.2 (559) | 73.8 (429) |
| LCDD (18) | 83.3 (15) | 77.8 (14) | 77.8 (14) | 55.6 (10) |
* SPE, UPE, serum IFE, urine IFE, FLC
** Data expressed as percent with number of cases in parenthesis
† Data from 1877 patients (Katzmann, 2009)
Summary
SPE alone is not an adequate screen for MM and AL amyloidosis. When patients have clinical symptoms of MM or AL amyloidosis, I recommend performing at least a SPE and serum FLC test. This will detect the vast majority of these patients. If these studies are negative, and there remains a strong clinical suspicion of MM or AL amyloidosis, IFE of serum, urine and bone marrow biopsy may be needed to detect cases with more subtle laboratory findings. If Waldenström’s macroglobulinemia is suspected, virtually all cases should be detected by the combination of SPE and serum FLC. Note that due to self-aggregation and hyperviscosity, some cases of Waldenström’s require further chemical treatment with reducing agents such as 2-mercaptoethanol. Lastly, asymptomatic patients (MGUS) with M proteins discovered serendipitously by SPE need to be evaluated for the quantity, heavy chain class and serum FLC content in their serum to better stratify their risk for progression to MM.

