New Concepts in Prenatal Testing: Sequential Integrated Screening
New Concepts in Prenatal Testing: Sequential Integrated Screening
 Today, several options are available for prenatal screening. In this review, I present background information on prenatal screening and the most recent addition to the testing—Sequential Integrated Screening, and put its use in perspective of other available tests. Sequential Integrated Screening offers a lower level of false positive tests than either the QUAD test or the First Trimester Screen, but slightly higher than the Full Integrated Screen. Sequential screening, however, provides information as early as the first trimester to the small number of women with very high risks (1:25 or greater) of carrying a fetus affected with Down syndrome. By doing so, its total detection of Down syndrome risk is as good as the 1st Trimester Screen or the Full Integrated Screen (detects 86% of Down syndrome cases), and about 60% of the Down syndrome cases will be picked up during the first trimester.
Today, several options are available for prenatal screening. In this review, I present background information on prenatal screening and the most recent addition to the testing—Sequential Integrated Screening, and put its use in perspective of other available tests. Sequential Integrated Screening offers a lower level of false positive tests than either the QUAD test or the First Trimester Screen, but slightly higher than the Full Integrated Screen. Sequential screening, however, provides information as early as the first trimester to the small number of women with very high risks (1:25 or greater) of carrying a fetus affected with Down syndrome. By doing so, its total detection of Down syndrome risk is as good as the 1st Trimester Screen or the Full Integrated Screen (detects 86% of Down syndrome cases), and about 60% of the Down syndrome cases will be picked up during the first trimester.
Common Defects
The three most common types of birth defects that may be detected by screening methods described in this review are neural tube defects, Down syndrome and Trisomy 18.
Neural Tube Defects are openings in the brain or spinal cord that occur early in fetal development. Some are not compatible with life (eg. anencephaly), whereas others (eg. spina bifida) may result in handicaps that range from mild to ones as serious as paralysis or mental retardation. Only neural tube defects not covered by skin (open spinal bifida — OSB) are detected by this screening method. Neural tube defects occur more often in individuals with previously affected children or with relatives who have had affected children. With recent attention to the importance of folic acid supplementation, there have been significant decreases in the prevalence of OSB-affected pregnancies.
Down Syndrome is a genetic abnormality specifically caused by an extra chromosome number 21. Fetuses with Down syndrome are spontaneously lost at a greater rate than unaffected fetuses during pregnancy. Individuals with Down syndrome are mentally retarded and can have serious medical problems such as heart defects. The degree of severity of the abnormalities can vary between affected individuals. The chances of having a baby with Down syndrome increase with the mother’s age, but in general, about one in 700 babies are born with Down syndrome.
Trisomy 18 is a genetic abnormality specifically caused by an extra chromosome number 18. Many fetuses with Trisomy 18 are spontaneously lost during pregnancy or at birth. The approximately 5% that survive for a year or longer after birth are usually severely mentally retarded and can have other serious medical problems. Once again, the chances of having a baby with Trisomy 18 increase with the mother’s age. It is, however, much less common than Down syndrome occurring about once in 6,000 newborns.
Screening versus Diagnostic Testing
When counseling the patient about this testing, it is important to emphasize that these are all screening, not diagnostic tests.
Diagnostic tests, such as cytogenetics performed on an amniocentesis or chorionic villus sampling (CVS) are tests that provide definitive information about a pregnancy with false positives and false negatives being very uncommon. Unfortunately, these diagnostic tests require an invasive procedure that carries a risk for losing the fetus and rarely may have complications for the mother.
Screening tests are performed in order that only women at relatively high risks for carrying an affected fetus are offered the potentially dangerous invasive diagnostic tests. The prenatal screening tests reviewed here require history, accurate gestational age determination, a blood sample from the mother to measure certain analytes (discussed below), and for the newer tests an ultrasound examination of the fetus by a specially trained ultrasonographer. The advantage of screening tests is that they do not carry the risk inherent with invasive tests. Careful pretest counseling is important so the patient understands the limitations of screening tests (false positives and false negatives) so that they will not be confused by the results.
Age as a Risk Factor
The original screening test was based upon the age of the mother. The risk for carrying a fetus with chromosomal abnormalities, especially Down syndrome and Trisomy 18, increases with the age of the mother. Down syndrome has an incidence of about 1 per 700 live births in all ethnic groups, but its incidence increases with age. Since the 1970s it has been standard to offer amniocentesis or CVS to women who are 35 years of age or older at the time of delivery. Yet, all pregnancies have a risk of carrying a Down syndrome fetus. Therefore, since most pregnancies occur in younger women, even though their risk is lower than that of women 35 years of age or older, most Down syndrome babies were products of the younger women who were not being offered the more definitive invasive diagnostic tests.
Second Trimester Screening Tests
These tests are part of sequential screening and are still needed (as the QUAD test) when a pregnancy is not known before week 14 of gestation
Alpha fetoprotein (AFP)
In the late 1980s, alpha fetoprotein (AFP) testing was offered to pregnant women because it was elevated in most women who were carrying a fetus with open spina bifida (OSB) and in almost all women who were carrying a fetus with anencephaly. At that time, the risk of OSB was about 1 in 1,000. However, since then, the strong association of folate deficiency and OSB has caused manufacturers to include folic acid in many food products and women who are planning on pregnancies often take folic acid supplements. As a result, current risk is about 1 in 1,700. The severity of this condition varies widely depending on where the spinal defect occurs. Today, we can detect about 80% of cases of OSB with second trimester testing for AFP. With the combination of better dietary measures and intervention, the CDC reported that the prevalence of OSB is about 1 in 5,200 live births.
While testing for OSB with AFP, it was discovered that the serum levels of alpha fetoprotein tended to be lower in women with Down syndrome pregnancies. This turned out to be a very weak screening test for Down syndrome detecting about 20% of Down syndrome cases in women younger than 35 years. However, at that time it was the only practical way to identify women younger than 35 years who might be at a high enough risk that it was worthwhile to offer them the invasive diagnostic testing, amniocentesis.
Triple Test (AFP, hCG and uE3)
The Triple test can detect about 70-75% of cases of Down syndrome with a 5% false positive rate (1 in 20 women will be positive). In the mid-1990s, it was reported that the addition of human chorionic gonadotropin (hCG) and unconjugated estriol (uE3) to AFP in addition to considering the patient’s age could detect about 65% of Down syndrome cases (Haddow et al. 1992, 1998). The hCG is a strong predictor of Down syndrome. In addition, uE3, turned out to be a useful marker for detecting Trisomy 18 (Edward’s syndrome), Smith-Lemli-Optiz syndrome (SLOS), and deficiencies of steroid sulfatase.One problem with this test was that all three analytes varied in concentration during the second trimester weeks that they were being tested (weeks 15-22.). Therefore, if there was an error in calculating the gestational age, such as due to an irregular menstrual period making calculation of the last menstrual period difficult, the patient may have been overestimated or underestimated for her risk of carrying an affected fetus. Because of this issue, it has been noted that accurate gestational age from ultrasound reporting gives more true positives and fewer false positives. For instance, Benn reported that the Triple test detects 66% of cases of Down syndrome (when ultrasound was used to determine gestational age, but only 57% when LMP was used) with a false positive rate of 5% (5.8% when LMP was used to determine gestational age). Because of its significantly poorer performance in detecting Down syndrome cases than the QUAD test, Warde Medical Laboratory no longer offers the Triple Test.
QUAD Test
The QUAD test can detect about 82% of cases of Down syndrome with a 5% false positive rate (1 in 20 women will be positive). It has become the standard and most used second trimester prenatal screen in clinical laboratories today. The addition of Inhibin A, producing the QUAD test, was especially helpful because the concentration of Inhibin A changes only minimally in concentration during the testing period. Its addition to the calculation of Down syndrome risk ameliorated somewhat the effect of misdating the pregnancy resulting in a significant reduction in false positives. With the QUAD test, Benn found that 73% (only 67% if LMP was used to calculate gestational age) of cases of Down syndrome were detected in women younger than 35, with a 4.6% false positive rate (5.5% if LMP was used). For the past decade, second trimester testing has been the generally accepted standard.
First Trimester & Full Integrated Screening Tests
First Trimester Test
The First Trimester Test can detect about 86 % of cases of Down syndrome with a 5% false positive rate (1 in 20 women will be positive). This test is available from week 11, day 0 through week 13, day 6 of gestation. The key factor that has allowed testing during the first trimester is the ultrasonic measurement of the Nuchal fold translucency (NT) thickness. It is by far, the strongest single predictor for estimating the risk of carrying a fetus with Down syndrome during the first trimester. The NT is the subcutaneous tissue between the cervical vertebrae and the skin. When the NT measurement is >95th percentile for a specific gestational age (determined by ultrasound dating), there is an increased risk for chromosomal abnormalities (especially Down syndrome). Similar to hCG, the MOM values increase in pregnancies affected by Down Syndrome such that about half of the values are above 2.0 MOM for each of those markers. However, NT is a much more effective marker than hCG because while about 8% of pregnancies from women not carrying a Down syndrome fetus will be above 2.0 MOM with hCG, only about 1.5% are above this point when NT is used as the marker. As a result, NT gives many fewer false positives (Figure 1A and 1B).
Figure 1A. hCG MOMs in unaffected pregnancies and those with Trisomy 21 (Down syndrome).
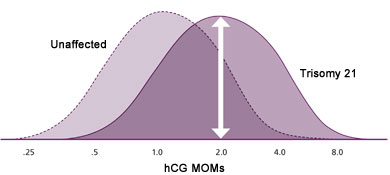
Figure 1B. NT MOMs in unaffected pregnancies and those with Trisomy 21 (Down syndrome).
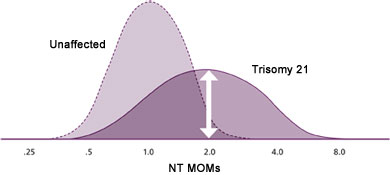
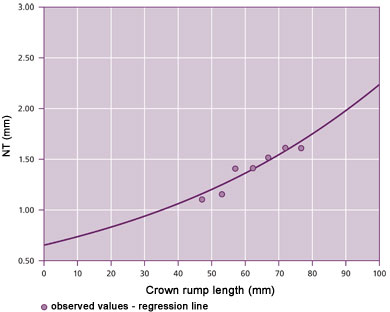
Before the risk estimate can be performed, we need to have documentation that the ultrasonographer is certified to perform NT measurements by one of the following: FASTER trial, Fetal Medicine Foundation (FMF) or Nuchal Translucency Quality Review (NTQR). To optimize our service, for an ultrasonographer that is new to our database, please contact Warde Medical Laboratory at 800 876-6522 and ask for the prenatal screening department prior to sending specimen. Palomaki et al (2008) recommend monitoring the consistency of information from sonographers by measuring 3 NT quality measures (medians, standard deviations and slopes). We record these data on our sonographers and work with the certifying agencies to assure quality measurements (figure 2).
When performing first trimester testing, use of ultrasound gestational dating is a strong predictor and its use is needed because PAPP-A is very sensitive to gestational dating error. PAPP-A levels increase in the late first trimester by about 50% per week, a much larger difference than seen with the test markers used during the second trimester. By using a combination of maternal age, ultrasound measurement of the nuchal fold translucency (NT), measurement of pregnancy-associated plasma protein A (PAPP-A), and hCG about 85% of cases of Down syndrome can be detected with about 5% false positives. Availability of an effective first trimester screen has allowed women to choose CVS as a diagnostic test.
Figure 2. Monitoring of NT versus crown-rump length measurements from a sample cohort – recommended increment is 15-25% per week. This data set shows a 17.3% increase in median NT per week.
The Full Integrated Screening Test
(Combined First and Second Trimester testing)
The Full Integrated Screen Test can detect about 87% of cases of Down syndrome with a false positive rate of about 1% (1 in 100 women will be positive). It requires the patient to have testing during both the first and second trimester. The first blood sample is obtained from week 11, day 0 through week 13, day 6 of gestation and a PAPP-A laboratory test is performed. Also, during the first trimester NT is measured by ultrasound as described above in the First Trimester Test. During the second trimester (weeks 15-22) the QUAD test is performed.
This test has the great advantage of the lowest false positive rate. Only 1 in 100 women will be positive. However, it requires two blood samples and the final results are not available until after the second trimester test. Therefore, women would not be able to avail themselves of the early cytogenetic testing at the end of the first trimester through CVS.
The Serum Integrated Screening Test
(Combined First and Second Trimester testing without NT measurements)
The Serum Integrated Screening Test can detect about 85% of cases of Down syndrome with a false positive rate of about 3.6% (1 in 28 women will be positive). Not all women have access to NT measurements, and in occasional cases, the NT measurements cannot be made. However, by performing PAPP-A testing once at any time during weeks 11, day 0 through week 13, day 6, and then the QUAD test in the second trimester, a woman who could not obtain an NT measurement will have about a 3% improvement in detection of Down syndrome and a decrease in the false positive rate from the QUAD test.
The Sequential Screen
(Combined First and Second Trimester testing with release of highest risk information)
Sequential Screening can detect about 86% of cases of Down syndrome with a false positive rate of about 2% (1 in 50 women will be positive). The Sequential Screen is a new option for prenatal testing that allows detection of Down syndrome or Trisomy 18 as early as the first trimester, and lowers false positive rates of the first trimester only test or the second trimester QUAD test from about 5-6 % to 2%.
It does this by combining the best features of the currently available first and second trimester tests. The Sequential Screen performs the same testing during the first and second trimester as the Full Integrated Screen. However, if the First Trimester test (performed as described in the First Trimester Test above) results in a risk of 1:25 or greater, the results are released right away, and the second trimester part of the test is not performed. Only about 1% of women will have this level of risk, but about 60% of the cases of Down syndrome will be detected at this relatively early time. Since only about 1 in 100 women will be positive with a risk as high as 1:25 in the first trimester, 99% of women will go on to have the second trimester tests performed as in the Full Integrated Screen. Therefore, the vast majority of women will go on to have the QUAD test performed in the second trimester, resulting in a false positive rate at about 2% while detecting about 86% of Down syndrome cases.
This test allows the detection of the highest risk pregnancies by the First trimester test as early as weeks 11-13, but keeps the false positive rate at less than half of the First trimester only test by deferring all the other cases until after the second trimester markers have been done.
Below, I’ve summarized our findings in a Table to allow individuals to see the advantages and disadvantages of each test. The highest detection rate with the lowest false positive rate is the Full Integrated Screen Test. The results can be available as soon as week 15 of the pregnancy and only 1 out of 100 women will be screen positive. The Sequential Screen offers the same testing, but if the result of the First Trimester part of the test shows a risk of 1:25 or higher, it will be immediately reported as a screen positive. About 60% of cases of Down syndrome will be found in these individuals and only 1 out of 100 women tested will be screen positive. However, because 99% of women will not have such a high risk, they will need to have the second trimester tests before their risk assessment can be given. If the patient wishes to have the information available by weeks 12-14, the First Trimester Test can provide a detection rate of about 86%. But for the 14 or 21 days earlier information, the false positive rate increases to about 5% (1 of 20 women will be screen positive).
Table. Comparison of Detection and False Positive Rates with Current Testing
| Test | When Performed (Weeks) | Date Available (Weeks) | Down syndrome Detections | False Positive Rate |
| QUAD | 15 – 22 | 15 -22 | 82% | 5% |
| 1st Trimester Test* | 11-13 | 11-13 | 86% | 5% |
| Sequential Screen** | 11 – 13 & 15 – 22 | High Risk 11-13 Lower Risk 15-22 | 86% | 2% |
| Full Integrated Screen* | 10 – 13 & 15 – 22 | 15 – 22 | 87% | 1% |
| Serum Integrated Screen* | 10 – 13 & 15 – 22 | 15 – 22 | 85% | 3.6% |
* Data averaged from the SURUSS and FASTER trials.
** Results on positives >1:25 available 1st Trimester, rest available after week 15. Data from Palomaki et al.
Key Questions about Prenatal Testing
1. How can I get a recalculation?
Just call Warde Medical Laboratory and give the information to the client services representative. If we need further information our technologists that specialize in the prenatal screening, or one of our pathologists will discuss the issue with you, your nurse or your staff, whichever you prefer.
2. My office was called for confirmation of information we already provided–why?
We call to confirm all screen positive cases before reporting them out. We have found that almost 20% of the time, a clerical error or omission has resulted in a false positive test.
3. One of my patient’s received a positive screen for Down syndrome and I have since performed an ultrasound that changes her gestational age by 6 days, can I get a recalculation of her result?
Ultrasound information is preferable on the initial sample, but, once the patient has been found to have a positive screen, we require a 10 day difference from the gestational age by LMP before we will change a positive screen for Down syndrome to a negative.
4. Why doesn’t Warde use Dried Blood spots for First Trimester testing?
Occasionally, we receive questions about the type of blood sample. Some clients note a convenience for using dried blood spots rather than serum for analysis. A recent independent comparison by Palomaki et al. (2007) of data from dried blood spots versus serum for first trimester analysis of PAPP-A and hCG found that the variances for these two analytes increased by 14% and 25% respectively in samples from dried blood spots. When translated to risk analysis, they found that the use of these dried blood spots resulted in a decrease in detection of pregnancies associated with Down syndrome pregnancies or an increase in the false positive rates. At Warde Medical Laboratory, we use fresh sera for these analytes.
Summary
Clearly, the state of prenatal screening continues to evolve. In the past 25 years we have gone from the AFP test designed mainly to detect open spina bifida to a QUAD test as the standard for second trimester testing. The First Trimester Screen allowed for cytogenetic studies to be performed during the end of the first trimester, but kept the false positive rate at about 5%. While the Full Integrated Screen decreased the false positive rate to about 1%, but no results were available until the second trimester.
The new Sequential Screen provides results during the first trimester for women with the highest risk (1:25 or greater) by the First Trimester Test, while keeping the overall false positive rate at about 2% by combing the First Trimester data with the QUAD test information to assure that fewer women are given false positive results than with First Trimester only testing.

