New Standard for Prenatal Screening – The Quad Test
The Quad Test is replacing the Triple Test as the standard prenatal screening procedure offered at Warde Medical Laboratory. This is because the Quad Test is able to detect more cases of Down syndrome than earlier screening methods while decreasing the false positive rate. In this article, I review the background on prenatal screening and the current use of the Quad Test at Warde Medical Laboratory.
About 3 percent of neonates suffer from birth defects, many of which are due to genetic abnormalities. Identifying pregnancies at greatest risk for having an affected fetus requires a combination of biochemical and clinical information.
Screening Tests vs. Diagnostic Tests
The Quad Test (like the Triple Test before it) is a screening test, not a diagnostic test. It is important that the patient understand this before she is tested. The diagnostic test to detect chromosomal abnormalities is cytogenetics, which requires either an amniocentesis or a chorionic villus sampling. These are invasive procedures and carry an inherent risk to the fetus and the mother. Therefore, to determine which women would benefit from offering the diagnostic testing, screening tests are used.
Screening tests do not establish that an individual has a given condition. Rather, they provide the clinician and genetic counselor with information to make an assessment of the probability that the condition exists. This type of assessment is useful, because, if the probability is high enough, it supports the use of invasive procedures to obtain material for the definitive chromosome studies.
Background on Screening Tests for Prenatal Risk
Maternal Age – Over 35
The first screening test that determined whether a woman would benefit from the invasive procedures was checking her age. Because chromosomal abnormalities (Down syndrome, Trisomy 18, etc.) occur with increased frequency with age (figure 1), women 35 years of age and older are offered amniocentesis to check for Down syndrome. However, since the vast majority of children are born to women younger than 35 years, the “35 years of age” screening test detected only about 25% of fetuses with Down syndrome. Furthermore, by offering the invasive procedures to women merely because they are at or over 35 years of age, the false positive rate is very high.
| Figure 1 |
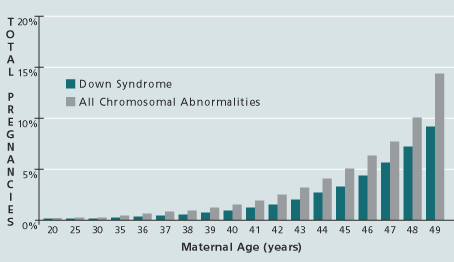 |
Women Younger Than 35
Alpha-fetoprotein (AFP) was the first marker to provide an indication that a woman younger than 35 might benefit from amniocentesis. AFP is the major serum protein of the fetus, synthesized by the fetal liver and yolk sac. It migrates from the fetal to the maternal circulation either by diffusing across the placenta, or by leaking from the kidney secretions into the amniotic fluid where it may diffuse across the amnion.
AFP levels in the maternal serum were (and still are) being used to detect open neural tube defects and anencephaly. In those conditions, the level of AFP (normally about 10,000-100,000 times higher in the fetus than in the maternal blood) increases in the maternal serum. This has proven to be an indicator that can detect about 80% (not all) of cases of open spina bifida. While studying this phenomenon, investigators learned that women carrying a Down syndrome fetus would have a decreased serum AFP level compared to unaffected pregnancies. By combining this information with the patient’s age, about 30-35% of cases of Down syndrome could be detected. While not an enormous improvement, it was the first way to detect women under the age of 35 years who might benefit from amniocentesis.
The Triple Test
The Triple Test was the next major advance in the screening test. It consisted of assaying the maternal blood during the second trimester for AFP, human chorionic gonadotropin (hCG), and unconjugated estriol (UE3). hCG is produced during pregnancy, first by the trophoblast and then in the placenta. hCG concentrations in the maternal serum during the second trimester are significantly higher in pregnancies with Down syndrome fetuses than in unaffected pregnancies.
The Triple Test also includes analysis of the maternal serum for unconjugated estriol (uE3). uE3 is a steroid product of the placenta, but which also requires the fetal liver to complete its synthesis. Similar to AFP, uE3 is reduced by about 25% in maternal sera from pregnancies affected by Down syndrome compared to unaffected pregnancies. In addition to augmenting the detection of Down syndrome and Trisomy 18 (Edward syndrome), measurement of uE3 can detect cases of steroid sulfatase deficiency that has been associated with prolonged labor.
Gestational Age and MOM
During the second trimester, levels of AFP and uE3 rise, whereas, hCG declines. Because of these changes of the analytes with time and because of method variation from laboratory to laboratory, results for the tests are expressed as multiples of the median value for that gestational day. For instance, if the laboratory has calculated that the median value for AFP of all their patients who have a gestational age of 17 weeks, 0 days is 40 ng/mL, and a particular patient has a value of 80 ng/mL she is exactly twice the median. Therefore, her multiple of the median (MOM) is 2.0 for AFP. This is performed for all 3 measurements. A pattern of relatively low AFP, low uE3 and high hCG increases the risk for having a Down syndrome fetus. However, that same pattern can result from a misdated gestation. This type of misdating occurs most often when the last menstrual period is used to estimate the gestational age, as opposed to ultrasound measurements taken after the fetus was 6 weeks, 0 days of age. Indeed, the study by Haddow et al. (1992) indicates that false positives can be reduced from about 7% to about 4% (using a 1:190 cut-off) by using ultrasonography for gestational age estimates. Although the Triple Test had limitations, it was able to detect about 60-65% of Down syndrome cases and about 80% of neural tube defects.
False Positive Rates
One major concern about the Triple Test is the relatively high false positive rate. Because the risk of Down syndrome increases with the age of the mother, the false positive rate increases with the age of the population being tested. Typical false positive rates using a cut-off of around 1:270 range from 7-9%. In the past few years we have seen a trend toward an increase in the number of older women having babies, a trend that statistically will increase the false positive rate. The addition of dimeric inhibin A (DIA) to this testing will lower the false positive rate slightly.
Dimeric Inhibin A (DIA) and the Quad Test
Dimeric Inhibin A (DIA) is a glycoprotein consisting of an alpha and beta A subunit. During pregnancy, it is mainly produced by the placenta and provides a negative feedback to the secretion of follicle stimulating hormone (FSH) by the anterior pituitary gland. Among patients carrying a Down syndrome fetus, DIA is increased to an average of 1.9 multiples of the median (MOM). However, unlike AFP, uE3 and hCG, the levels of DIA are relatively constant throughout the second trimester testing period, which minimizes the effect of gestational age estimates.
In studies at other centers, the addition of Inhibin A to the Triple Test has increased the detection of Down syndrome as much as 8%. In addition to the benefit of increased detection, there is also a slight reduction of the false positive rate by adding DIA to the Prenatal Screen panel as the Quad Test. Indeed, Dr. Cannick’s laboratory in Rhode Island (one of the originators of the Quad Test) claims a detection of almost 80% of cases of Down syndrome with the addition of the DIA marker. Their recent statistics show that the odds of being affected given a positive result with the Quad Test are 1 in 50 (with the Triple Test this number was 1 in 63).
Clinical Information Needed for Quad Test Risk Estimate
Down syndrome (trisomy 21) has an incidence of about 1.3/1000 births.
The Quad Test may be performed between 15 and 22 weeks of gestation (with 16-18 weeks being considered optimum).
Several factors influence the results, therefore, it is of considerable importance for the clinician to provide the laboratory with information about the patient to permit an appropriate interpretation of results.
The gestational age is important because AFP, hCG and uE3 levels change during pregnancy. Therefore, if the pregnancy is really in its 18th week and the laboratory is told the pregnancy is in its 16th week, the value obtained for AFP will appear to be higher than expected for that time in the pregnancy.
If an ultrasound was performed after the fetus was 6 weeks, 0 days of age, please provide it. Haddow and others have found significantly fewer false positive results when such ultrasound information is used in preference to last menstrual period.
Fortunately, DIA does not change as much during the pregnancy and its inclusion in the Quad Test ameliorates the gestational age effect of the other quantified analytes.
Several other factors affect the MOM. The maternal weight is inversely related to the concentration of AFP, hCG, uE3 and DIA. Therefore, the maternal weight must also be included in our calculation for an accurate estimate of risk.
Race is another important factor, with Blacks having AFP approximately 10% higher than Whites. Diabetes (insulin-dependent—not gestational) is also known to result in AFP values that are about 40% lower than those of nondiabetic mothers. Therefore, information on weight, insulin-dependent diabetes and race must be included for an accurate MOM to be obtained.
Individuals with a twin pregnancy have higher MSAFP levels than those carrying a single pregnancy. However, even in twin pregnancies, MOMs greater than 4.0 are of concern and are associated with increased risk of low birth weight, prematurity and the possibility of a neural tube defect. A common error we find is staff recording a woman who has a previous child as having a multiple pregnancy. To avoid this, we prefer the use of “single”, “twin” and “triplet” on the forms rather than the term “multiple gestation”.
Key Questions about Prenatal Testing
- How can I get a recalculation? Just call Warde Medical Laboratory and give the information to the client services representative. If we need further information our technologists that specialize in the Quad Test, or one of our pathologists will discuss the issue with you, your nurse or your staff, whichever you prefer. Client Services 1-800-760-9969.
- Why do you prefer to use the ultrasound date versus the last menstrual period date? As mentioned above, a weakness of the prenatal testing is the relatively high percentage of false positive tests. The most definitive work on this subject was published by Haddow et al. in the New England Journal of Medicine. Table 1 shows key data from their work documenting a dramatic decrease in the false positive rate when the ultrasound was used instead of the last menstrual period (LMP). No studies currently published show LMP to produce a lower false positive rate than ultrasound data.Table 1
More False Positive with LMP than US Date
for Down Syndrome (1 in 190) False Positive Percent
Initial Dating MethodSite 1Site 2TotalUltrasound2.44.63.7LMP8.78.68.7Total5.77.16.6 - My office was called for confirmation of information we already provided—why? We call to confirm all screen positive cases before reporting them out. We have found that almost 30% of the time, a clerical error or omission has resulted in a false positive test.
- One of my patients received a positive screen for Down syndrome and I have since performed an ultrasound that changes her gestational age by 9 days, can I get a recalculation of her result? Ultrasound information is preferable on the initial sample, but, once the patient has been found to have a positive screen, we require a 14 day difference from the gestational age by LMP before we will change a positive screen for Down syndrome to a negative. This reflects midtrimester ultrasounds that have an accuracy of plus or minus 7 days (1 Standard Deviation). The 95% confidence limit (2 Standard Deviations) requires 14 days.
References
- Cuckle H. Biochemical screening for Down syndrome. Eur J Obstet Gynecol Reprod Biol 2000;92:97-101.
- Debieve F, Bouckaert a, et al. Multiple screening for fetal Down syndrome with the classical Triple Test, dimeric inhibin A and ultrasound. Gynecol Obstet Invest 2000;49:221-226.
- Haddow JE, Palomacki GE, et a. Prenatal screening for Down syndrome with use of maternal serum markers. NEJM 1992;327:588-593.
- Haddow JE, Palomaki GE, et al. Second trimester screening for Down syndrome using maternal serum dimeric inhibin A. J Med Screen 1998;5:115-119.
- Lambert-Messerlian GM, Canick JA, et al. Second trimester levels of maternal serum inhibin A, total inhibin, alpha inhibin precursor, and activin in Down syndrome pregnancy. J Med Screen 1996;3:58-62.
- Wenstrom KD, Owen J, et al. Alpha-fetoprotein, free beta-human chorionic gonadotropin, and dimeric inhibin A produce the best results in a three-analyte, multiple-marker screening test for fetal Down syndrome. Am J Obstet Gynecol 1997;177:987-991.
- Wenstrom KD, Chu DC, et al. Maternal serum alpha-fetorotein and dimeric inhibin A detect aneuploidies other than Down syndrome. Am J Obstet Gynecol 1998;179:966-970.
- Yoshida K, Kuwabara Y, et al. Dimeric inhibin A as a fourth marker for Down syndrome maternal serum screening in native Japanese women. J Obstet Gynaecoll Res 2000;26:171-17
Quad Test CME Questions
Answers Question 1. The Quad Test consists of:- alpha fetoprotein, unconjugated estriol, human chorionic gonadotropin and alpha human chorionic gonadotropin
- pregnancy associated protein-A, unconjugated estriol, human chorionic gonadotropin and alpha human chorionic gonadotropin
- pregnancy associated protein-A, dimeric inhibin A, human chorionic gonadotropin and alpha human chorionic gonadotropin
- alpha fetoprotein, unconjugated estriol, human chorionic gonadotropin and dimeric inhibin A
- pregnancy associated protein-A, unconjugated estriol, human chorionic gonadotropin and fetal albumin
- estimation of fetal age by ultrasound
- estimation of fetal age by last menstrual period (LMP)
- estimation of fetal age by uterus size on physical examination
- estimation of fetal age by pelvic X-ray
- estimation of fetal age by the ratio of analytes in the Quad Test
- maternal age, maternal weight, maternal race, history of gestational diabetes
- maternal age, maternal weight, maternal race, history of insulin-dependent diabetes
- paternal age, maternal age, maternal weight, maternal race
- paternal age, maternal age, maternal weight, history of insulin-dependent diabetes
- paternal age, maternal age, maternal weight, maternal race, history of gestational diabetes
- Mel Brooks
- Dick Shawn
- Gene Wilder
- Zero Mostel
- Harvey Korman

