Pain Management
Introduction
Reports on the prevalence of chronic pain in America ranges anywhere from 3% to 40% 1. This far exceeds diseases such as diabetes (23.6 million) 2 and heart attack (23.2 million) 3 put together. Yet, the National Institutes of Health (NIH) spends more resources on diabetes and heart attacks than chronic pain. It has been reported that the NIH spends less than 1% of its resources on chronic pain 4. Consequently, many patients with chronic pain are under diagnosed and under treated because physicians increasingly fail to provide comprehensive pain treatment. Reasons for this include: fear of patient misuse of prescription drugs leading to diversion, abuse, addiction, personal biases, fear of law suits or even inadequate physician training in pain management 5. It is also recognized that medical students and residents receive inadequate training in these techniques, and practitioners are not very familiar with applications and implications of Urine Drug Testing (UDT). The overall consequence of this is a loss in productivity, personal income and quality of life of pain patients.
Treatment for Chronic Pain
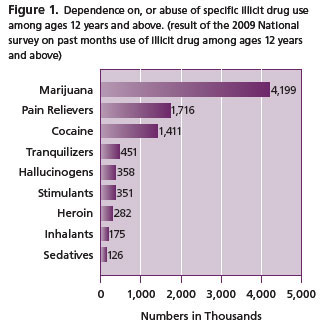
Recognizing the need for urgent pain treatment, coupled with the potential for abuse of pain medications in an environment of illicit drug abuse/misuse (Figure 1), the Panel on Chronic Non-Cancer Pain (CNCP) among others 6, 7, have recommended treatment modalities involving opioids. One of the CNCP recommendations states that, opioid treatment should be monitored by UDT, and possibly reassessed at a later date.
Opioids
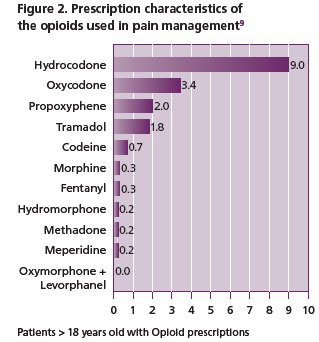
In the setting of pain management, approximately 90% of patients receive opioids, of which the most prescribed is hydrocodone (Vicodin) as shown in Figure 2 8, 9. Opioids are classified as natural opiates (morphine and codeine derivative of opium), semi-synthetic (such as oxycodone, hydrocodone, oxymorphone, buprenorphine and hydromorphone, derived from opiates) or synthetic (man made such as fentanyl, methadone).
 They are powerful and effective analgesics which make them useful in the management of moderate to severe chronic pain. When ingested, opioids yield metabolite (Table 1) detectable in urine, some of which are active. Thus, urine serves as a reservoir of information for ingested drugs, more readily available compared to other specimens.
They are powerful and effective analgesics which make them useful in the management of moderate to severe chronic pain. When ingested, opioids yield metabolite (Table 1) detectable in urine, some of which are active. Thus, urine serves as a reservoir of information for ingested drugs, more readily available compared to other specimens.
Specimen Types for Drug Testing
Apart from urine, specimens utilized for drug testing include hair, serum/plasma, saliva, sweat and nails. Urine is the specimen of choice for drug testing since some drugs reach it within an hour when smoked or injected. Orally ingested drugs reach the urine much slower (couple of hours). As an ultrafiltrate of blood, urine is continuously produced by the kidneys; making it readily available and easy to collect. It consists of excreted products from the body including ingested parent drugs and/or metabolites, excess water and salts, creatinine and urea. Filtered essential components required for body functions are reabsorbed by the kidneys.
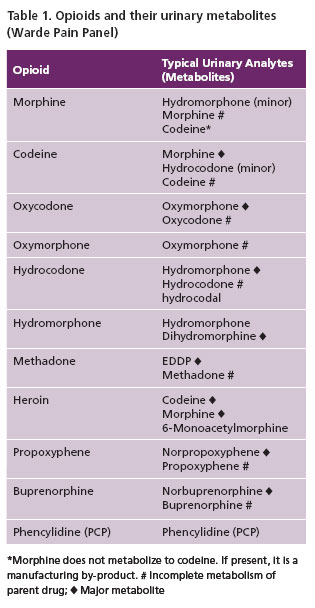 The daily composition and volume of urine is variable (1–2 liter/24hours) depending on environmental factors, diet, health, fluid intake and effect of drugs. For many opioids, parent drug and or metabolite(s) are available in urine (Table 1), allowing for a longer window of detection than for serum or saliva (Table 2, 3). Thus UDT is generally accepted as the gold standard for drug testing. Issues related to different specimen types are provided in Table 2.
The daily composition and volume of urine is variable (1–2 liter/24hours) depending on environmental factors, diet, health, fluid intake and effect of drugs. For many opioids, parent drug and or metabolite(s) are available in urine (Table 1), allowing for a longer window of detection than for serum or saliva (Table 2, 3). Thus UDT is generally accepted as the gold standard for drug testing. Issues related to different specimen types are provided in Table 2.
Historical Aspects of UDT
1. Regulation of UDT began after an increase of alcohol and drug (marijuana) related deaths occurred aboard the USS Nimitz in the early 1980s. This prompted the U.S. military to introduce a “zero tolerance” random drug testing policy. Subsequently, illicit drug use dropped from 30% to 5% in the military 11. Prior to this period, UDT was used mainly in emergency rooms, drug treatment facilities and in the criminal system.
2. Less than a decade later (1986), President Reagan issued an Executive Order Requiring a Drug Free Federal Workplace. This mandated all agencies to establish a program to test for drug exposure of federal employees in positions regarded as “sensitive”. The program also mandates drug testing for individuals involved in accidents, and for all new job applicants 12.
3. In 198713 the Department of Transportation (DOT) enacted a similar drug free program.
4. Since then, UDT has become common in schools, pre-employment physicals, addiction medicine and is becoming commonplace in pain management.
 Rational for Urine Drug Testing (UDT) in Pain Management
Rational for Urine Drug Testing (UDT) in Pain Management
Several studies have shown that patients on opioid treatment are more likely to misuse both prescribed and non-prescribed drugs (up to 40%) than they are to become addicted (up to 23.4%) 1, 14.
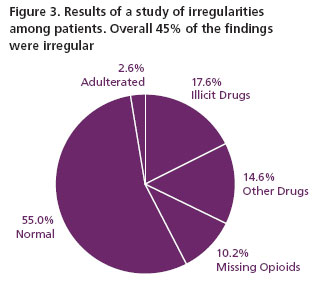
A 2007 study involving 470 patients attending a pain management program found substantial irregularities (Figure 3) including attempts to adulterate urine samples 15.
Adherence monitoring has been shown to decrease controlled substance abuse and illicit drug use as demonstrated by the military 10. To this effect, the goal of UDT for patients on opioid treatment should be two-fold 16:
- To ensure compliance i.e. confirm recent use of prescribed drugs (principally opioids and adjuvant drugs such as depressants e.g. benzodiazepines and stimulants).
- To exclude the presence of non-prescribed or illicit drugs e.g. cocaine, marijuana and amphetamine (Table 3).
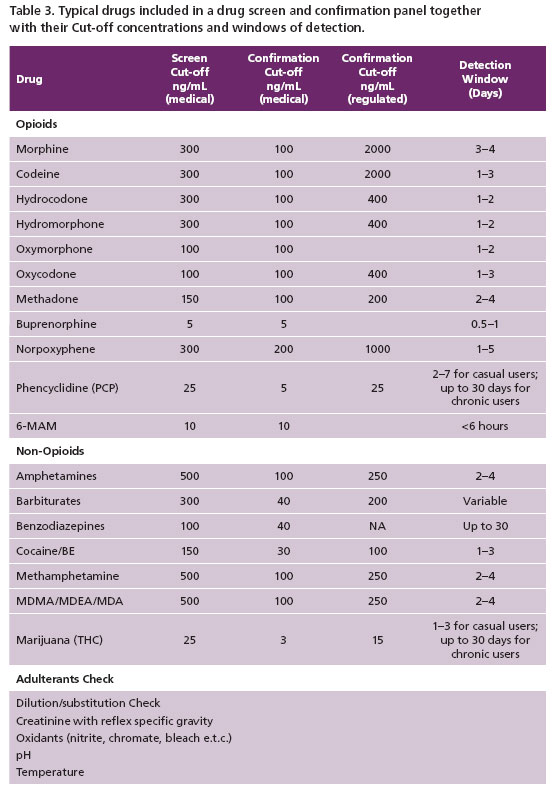
Screening Panel for UDT
In order to provide the appropriate service for drug adherence monitoring, the toxicology laboratory at Warde Medical Laboratory provides a screening panel that reflects commonly prescribed opioids as well as adjuvant drugs likely to be prescribed. Table 3 shows an example of such a drug screen panel. This profile can be modified by additions or deletions of drugs to be tested depending on patient history, treatment goals (based on consultations with physicians), prevalence of abused drugs in the geographical region, cost, and other factors. The laboratory cut-offs (Table 3) are selected to be sensitive enough to detect drugs of interest and their metabolites, avoiding false negatives and positives; consistent with prescribing physician expectations.
Laboratory Methodologies used in UDT
Immunoassay
Enzyme Immunoassay (EIA) is used for the initial evaluation of samples for the presence or absence of a drug group e.g. the opioids or the benzodiazepine group of drugs. EIA is very fast to perform, cost effective and requires less technical skills than performing mass spectrometry. EIAs for opioid testing are designed to be sensitive and specific enough to quickly identify any samples containing eithe rmorphine, codeine or both drugs. Because of their poor specificity, EIA positives are only presumptive and require confirmation testing for identification of specific drugs and or their metabolites in a urine sample (Table 1). The most dependable confirmatory methodologies in a toxicology laboratory are based on chromatographic methods e.g. mass spectrometry.
Mass Spectrometry
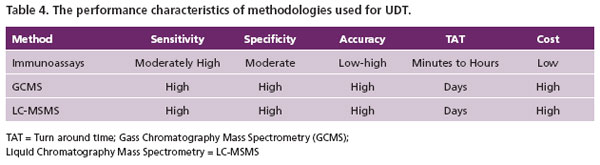
Drug confirmation testing at Warde Medical Laboratories is based on either gas chromatography mass spectrometry (GCMS) or liquid chromatography tandem mass spectrometry (LC-MSMS). These methodologies are usually much more sensitive and specific (equivalent to the “fingerprint” of an analyte) than immunoassays. Their specificities allows for the unequivocal simultaneous detection of several opioids and or their metabolites within the opioid class if present in the sample (Table 1). If properly validated, the high specificity and sensitivity eliminate false-positive or negative results. Mass spectrometry is considerably more sensitive than most EIAs. This is important in pain management because if the prescribed drug or its metabolite is ingested, it must be present and detected at much lower levels than used for the EIA cut-off value. However, mass spectrometric methods are expensive, require highly trained personnel and longer turn around times (TAT) relative to EIA. The performance characteristics of both the screen (EIA) and confirmation methods (Mass Spectrometric) are presented in Table 4.
Specimen Validity Testing
Patients with chronic pain who abuse drugs are aware of the consequences of their actions, which include removal from the treatment program. These patients are also knowledgeable about methods of adulteration which if undetected, will conceal the non-prescribed drugs in their urine samples. They are, therefore, motivated to adulterate their urine to avoid being caught for abused drug use (Figure 3). Some adulteration methods used by patients include:
Dilution. The patient achieves this by drinking large amounts of water (in vivo adulteration), or adding liquids to their urine sample (in vitro adulteration). A positive result in dilute urine is relatively easy to interpret contrary to a negative result in dilute urine.
Substitution. Some patients attempt to substitute their urine with another person’s urine, synthetic urine purchased through the Internet or even animal urine.
Chemicals. Another method of adulteration is the addition of chemicals (bleach, soap, salt) to the urine sample, which if undetected, are capable of interfering with the detection method. It is, therefore commonplace for the UDT laboratory to include a chemical detection mechanism in the screening procedure, for the purpose of isolating samples that have been tampered with by the patient in order to conceal detection of abused drugs 17.
The laboratory will reject adulterated samples and advise the client about a possible recollection with supervision as appropriate. As such, the validation of a urine sample should be initiated at the collection site, by noting the temperature of the sample within 4 minutes of the collection. The temperature should be between 90ºC to 100ºC for a freshly collected urine sample. The pH should also be noted. Normal urine pH is 4.5 to 9.0. Adulteration with chemicals, as well as bacterial infection will drift the pH outside this range. The laboratory also verifies that salts have not been added to the urine samples, to make up for a dilution as explained above. This is usually done by measuring the specific gravity of the urine as well as its creatinine level. All of these factors can complicate UDT result interpretation.
Interpretation of UDT Results
In pain management, the ingested prescribed drug and or its metabolite are expected to be present and detected (true positive); this means that its concentration is above the stipulated medical cut-off value. The cut-off is the concentration at which a target drug is determined to be present and detected (true positive) or absent, not detected (true negative). The cut-off value is chosen based on the purpose for which it’s being used e.g. whether for forensic or medical reason.
For example, the federally regulated confirmation cut-off for non-medical morphine is set at a high level of 2,000 ng/ mL because in patients abusing these medications the levels obtained are usually higher (Table 3, regulated cut-offs). This cut-off is inappropriate for medical situations where the objective is to detect the prescribed drug and absence of non-prescribed drugs. For medical purposes therefore, the cut-off is usually set to a low value, to allow detection of traces of any drugs present. If the medical (non-regulatory) cut-off for morphine is set at 100 ng/mL for example, positive results will be expected to be at least 100 ng/mL. A result below the cut-off e.g. 99 ng/mL will be reported as negative/none detected. Several reasons exist for seeing such a result (below the cut-off concentration).
These include:
- non-compliance
- over hydration
- inappropriate collection time
- diversion.
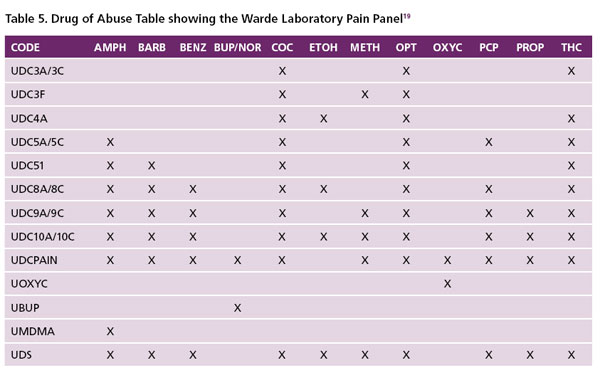
False Negatives. A false negative may be caused by a number of factors including clerical errors, patient tampering with their urine sample (e.g. substitution with another persons drug free urine, synthetic urine, animal urine, self catheterization), or the laboratory cut-off is too high, above the low urinary concentration of the drug or metabolite. Co-administration of over the counter and prescription drugs (e.g. naloxone and dextromethorphan) have been reported to cause negative opioid results 18. False negative immunoassay results can also occur by adulteration of the urine sample with chemicals such as nitrite, pyridine, chromate, halogens, or glutaraldehyde. In addition, false negative results can occur if the ordering physician picks the wrong panel (i.e. a panel not containing the drug of interest). Table 5 (the Warde Medical Laboratory Clinical Drug of Abuse Panels) shows options available to the ordering physician 19. For pain management, the correct panel to pick from this list will be the Code: UDCPAIN. This panel has been adapted to detect oxycodone and buprenorphine. Unlike most of the other opioids, buprenorphine has less potential for abuse. Buprenorphine does not provide the same levels of pleasure and positive mood elevation, nor does it cause cravings as strong as commonly abused opioids such as heroin, morphine, oxycodone, hydrocodone and others. As such, physicians are prescribing it more frequently for pain treatment, and will like to monitor compliance.
Inappropriate EIA choice and design will miss some opioids, particularly the synthetic opioids and oxycodone; consequently, a confirmatory test will not be reflexed for further testing, hence a false negative result will be reported. A complete absence of a drug (negative) will be a result less than the detection limit of the method (i.e. the sensitivity limit of the method), which may vary from one laboratory to another laboratory depending on the methodology used. Therefore, whenever in doubt about
i. a patient’s negative result
ii. an unexpected positive result or
iii. which panel to order,
the health care provider should promptly contact the laboratory to ensure that the drug of interest is reliably identified. When the patient is a reliable historian and the health provider expects a positive result, a no “cut-off” testing should be requested. For that request, the laboratory will report any values detected, from the sensitivity limit to and above the cut-off value of the test, the so called “no tolerance” policy of the health care provider. This will generate a report with a quantitative value of the drug to the lowest level detectable by the method used.
False Positive. False positives can occur where the test indicates a positive when the target drug is actually absent. Cross reactants (analytes similar or dissimilar in structure to the target, reacting with an immunoassay lacking sufficient
specificity), are a possible source of such false positives. Other causes are clerical errors, sample mix-up, sample mislabeling and technical errors. A very low cut-off is also a source of frequent false positives. The toxicology laboratory will usually select an immunoassay with the specificity and selectivity it requires to avoid false positives. Detection in urine of non-prescribed synthetic opioids such as fentanyl and oxycodone which are not known metabolites of any opioids, indicates their ingestion by the patient.
Pseudo-false positive. A pseudo-false positive can occur and such results require a careful study of patient medication before any conclusive interpretations are made about drug abuse. This situation is observed with drugs that have active metabolites. The detection of hydromorphone in the urine of a patient prescribed hydrocodone or morphine for example may appear to indicate abuse of hydromorphone. However, hydromorphone is a metabolite of both drugs and can appear in the urine of this patient. In an ultra fast metabolizer of hydrocodone, it is possible that only hydromorphone will be detected in the urine without any traces of hydrocodone. Another example of a pseudo-false positive result is the detection of oxymorphone only or together with some oxycodone, when only the latter is ingested. If the patient is on oxymorphone and you see noroxycodone, that person has ingested oxycodone in place of or in addition to oxymorphone. Knowledge of drugs and their metabolites is required for accurate interpretation of UDT results (Table 1). Detection of drugs other than the prescribed drug or its metabolite represents either abuse/misuse, or a contaminant (Table 1 shows opiods and their urinary metabolites). The laboratory is always available to assist with interpretation issues.
From the ongoing discussion, it is obvious that the interpretation of UDT is complex. Therefore knowledge of patient history is essential for a complete objective interpretation of UDT results.
Correlation between dose ingested and urinary drug levels
A frequently asked question by health care providers is whether there is a correlation between the dose ingested and concentration of the drug in urine. The simple answer is that, considering the pharmacokinetic and pharmacologic variables involved, it is difficult to establish a correlation between the amount of drug ingested (dose), and the amount detected in urine. The amount of drug and or metabolite(s) detected in urine (unlike in serum/plasma), cannot be used to make specific determinations regarding proper dosing, or in some cases the amount of drug ingested. UDT cannot indicate the amount of drug taken, when the last dose was administered or the source of the drug because the inter-subject variation of drugs in urine is high. Variations in urine concentration of 620 ng/mL to 3160 ng/mL amphetamine have been reported in two individuals each administered a dose of 5 mg/mL of the drug 20. Correlation between the dose of a drug and the amount detected is valid for blood drug levels only after a “steady state” concentration has been reached 21. Another frequently asked question is whether passive inhalation of cannabis or cocaine can cause a positive drug test. Published data indicate that passive inhalation cannot be the cause of positive UDT result 22.
Conclusion
For proper management of the pain patient, UDT is a very resourceful tool. It augments self reporting which
is very unreliable. However, the interpretation of UDT results can be challenging for persons unfamiliar with drug metabolism. For all interpretative questions, the staff at Warde Medical Laboratories is always available to provide the appropriate assistance.
References
- Trescot, AM., Boswell MV., Atluri SL., Hansen HC., Deer TR., Abdi S., Jasper JF., Singh, EA., Johnson BW., Cicala RS., Dunbar EE., Helm S., Varley KG., Suchdev PK., Swicegood JR., Calodney AK., Ogoke BA., Minore WS. and Manchikanti L. Opioid guidelines in the management of chronic non-cancer pain. Pain Physician. 2006; 9:1–39
- American Diabetes Association. http://www.diabetes.org/diabetes-basics/diabetes-statistics
- American Heart Association. http://www.americanheart.org/downloadable/heart/1240250946756LS-1982%20Heart%20and%20Stroke%20Update.042009.pdf.
- The May Day Fund, Meeting held in Washington D.C. June, 2009 Data on specific disease/research area spending for 2008 is available through the National Institutes of Healths Research Portfolio Online Reporting Tool (RePORT). Bethesda, M: NIH; 2008 [cited July 30, 2009. http://www.report.nih.gov/ nihdatabook/Carts/SlideGen.aspx?chartid=4
- Gary MR, Roger B., Robert LB., Fern W., and George W. Urine drug test interpretation: What do physicians know? J. Opioid Management. 2007; 3: 80–85.
- The WHO. www.who.int/cancer/palliative/ painladder/en/
- Chou, R. et al. Opioid Treatment Guidelines. J. of Pain, 2009; 10 :113–130
- Tellioglu, T. The use of Urine Drug Testing to Monitor Patients Receiving Chronic Opioid Therapy for Persistent Pain Conditions. 2008; 91: 279–282.
- Arvind N. and Richard AB. A Healthcare Claims Database Analysis to Estimate the Prevalence of Chronic Opioid Use by Adult Patients in the United States. Presented at the 25th Annual Meeting of the American Academy of Pain.
- Rouen, D., Dolan, K. & Kimber, J. (2001). National Drug and Alcohol Research Centre. University of New South Wales, Sydney.
- Bay RM., Marsden ME. Rachal JV. Peterson, MR. Drugs in the military workplace: Findings from the 1988 World wide survey. NDA Res. Monogr 1990; 100: 25–43
- Reagan R. Executive order 12564. Federal Register. Vol. 5, No.180. September
- Levine MR., Rennie WP. Pre-employment urine drug testing of hospital employees: Future questions and review of current literature. Occup Eniron. Med 2004; 61: 318–324.
- Fishbain DA, Cole B, Lewis J, Rosomoff HL, Rosomoff RS. What percentage of chronic nonmalignant pain patients exposed to chronic opioid analgesic therapy develop abuse/addiction and/or aberrant drug-related behaviors? A structured evidence-based review. Pain Med. 2008; 9:444–459.
- Michna E et al. Urine Toxicology Screening Among Chronic Pain Patients on Opioid Therapy: Frequency and Predictability of Abnormal Findings. Clin J Pain, 2007;23:173–179.
- Cone EJ, Caplan YH, Black DL, Robert T, Moser F. Urine drug testing of chronic pain patients: licit and illicit drug patterns. J. Anal Toxicol. 2008; 32:530–543.
- Coleman, DE. & Baselt, RC. Efficacy of two commercial products for altering urine drug test results. Journal of Toxicology — Clinical Toxicology, 1997; 35: 637–642.
- Storrow AB, Wians FH Jr, Mikkelsen SL, Norton J. Does naloxone cause a positive urine opiate screen? Ann Emerg Med. 1994; 24: 1151–1153
- Warde Medical Laboratory. 2011 Directory of Service (in Appendices)
- Poklis, A, Still, J., Slattum, PW. Edinboro, LF., Saady, JJ. & Costantino, A. Urinary excretion of d-amphetamine following oral doses in humans: Implications for urine drug testing in humans. J. of Anal. Toxicology, 1998; 22: 481–487.
- Galloway JH, Marsh ID. Detection of drug misuse; an addictive challenge. J Clin Pathol. 1999; 52:713–718.
- Cone, E.J. Marijuana effects and urinalysis after passive inhalation and oral ingestion. In: Research Findings of Abused Substances. C.N. Chang and R.L. Hawks, Eds. NIDA Research Monograph 99, pg 88–96, 1990.

