Revised Criteria for the Diagnosis of Hepatitis C Infection
In May of this year, the Centers for Disease Control and Prevention (CDC) issued revised guidelines for the diagnosis of hepatitis C virus (HCV) infection, largely in response to the discontinuation of the CHIRON RIBA test, currently the only commercially available recombinant immunoblot assay (RIBA) for supplemental testing following detection of HCV antibodies on initial ELISA screen. The lack of availability of this key supplemental test prompted changes to the CDC’s diagnostic algorithm that may affect clients of Warde Medical Laboratory.
The previous approach was based on the enhanced specificity of RIBA in determining the presence of antibodies to proteins unique to HCV for those samples that yielded relatively low signal intensity on initial reactive ELISA screens. The new algorithm utilizes nucleic acid testing (NAT) for all positive screens in lieu of a supplemental antibody detection method for low-positive screens. There are advantages and disadvantages to the new approach. An advantage is the “gold standard” status of NAT for the determination of current HCV infection — NAT is extremely sensitive and a positive HCV NAT has extremely high predictive value for current HCV infection in the setting of an initial positive ELISA screen.
One potential disadvantage is the need to submit a separate sample for NAT, since the extreme sensitivity of NAT (and hence the prevention of sample contamination) precludes the use of a sample that has been tested previously by other methods.
Another potential disadvantage is that antibody screening (by either ELISA or RIBA) and NAT are fundamentally different tests that measure different things. The goal of ELISA and RIBA is to detect host immune response to the infection, whether the infection is active or not. The goal of NAT is to detect the virus itself. In the case of certain other infectious agents (for instance, HIV) these goals are essentially similar, since spontaneous clearance of HIV does not occur, and an untreated patient with active infection will harbor both anti-HIV antibodies and HIV nucleic acid. In the case of HCV, however, approximately 15 to 25% of individuals who are initially infected with the virus will clear it spontaneously (through normal immune mechanisms), but will still harbor anti-HCV antibodies. Hence, it remains difficult to distinguish a false-positive ELISA screen from a situation in which an individual contracted, and then cleared, an HCV infection.
According to the CDC, if there is clinical importance to distinguishing past resolved HCV infection from a biologic false positive HCV antibody screen (i.e. cross-reactivity of naturally occurring patient antibodies to the anti-HCV reagent), then testing with an alternative HCV antibody screening platform may be considered. As always, if there is clinical suspicion for exposure to HCV, whether in the setting of a positive or negative ELISA screen, repeat testing should be considered since antibodies may not be detected for approximately 12 weeks after initial exposure.
The revised algorithm, including Warde test codes, is provided here.
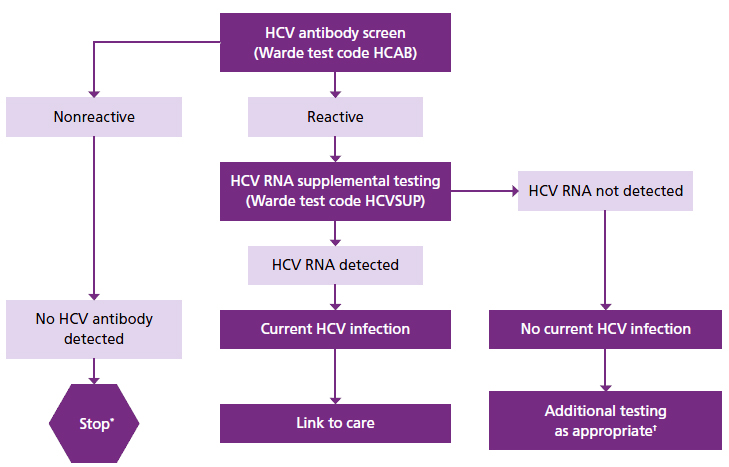
* For persons who might have been exposed to HCV within the past 6 months, testing for HCV RNA or follow-up testing for HCV antibody is recommended. For persons who are immunocompromised, testing for HCV RNA can be considered.
† To differentiate past, resolved HCV infection from biologic false positivity for HCV antibody, testing with another HCV antibody assay can be considered. Repeat HCV RNA testing if the person tested is suspected to have had HCV exposure within the past 6 months or has clinical evidence of HCV disease, or if there is concern regarding the handling or storage of the test specimen.

