Screening Methods for the Diagnosis of Cushing’s Syndrome
Introduction
Cortisol is the major glucocorticoid hormone produced by the human adrenal cortex. It is derived from cholesterol (either from the diet or de novo synthesis from acetate) through a series of enzymatic reactions of which 21-Hydroxylase is the most critical (1). Evidence of hypersecretion of cortisol is the criterion for the diagnosis of Cushing’s syndrome (CS), a relatively uncommon disease. It is estimated that each year 10 to 15 per million people mainly adults aged 20-50 are affected, particularly women.
Function
Cortisol is multifunctional with respect to its target organs; it is involved in the maintenance of electrolyte balance and blood pressure, regulation of carbohydrate, protein and lipid metabolism as well as immune response suppression (2). Patients diagnosed with Addison’s disease (hypocortisolism) are usually administered physiological doses of cortisol as a replacement therapy. Cortisol is also used for the treatment of allergic disorders, neoplastic diseases, skin disease, respiratory system diseases, nephrotic syndrome and inflammatory disorders. Thus, it is important to be able to measure cortisol levels in individuals suspected of Cushing’s syndrome (CS) as opposed to pseudo-Cushing’s (conditions associated with increased cortisol production, e.g., alcoholism and surgery, but with fewer clinical signs and symptoms than true Cushing’s syndrome).
Control and regulation of release of Cortisol
In order to make an accurate assessment of cortisol levels in the laboratory, it is important to recognize that cortisol secretion is highly regulated and, therefore, the timing of sample collection is crucial. Corticotropin-releasing hormone (CRH) from the hypothalamus and adenocorticotrophic hormone (ACTH) from the pituitary regulate the release of cortisol from the adrenal glands referred to as the hypothalamic-pituitary-adrenal axis (Figure 1).
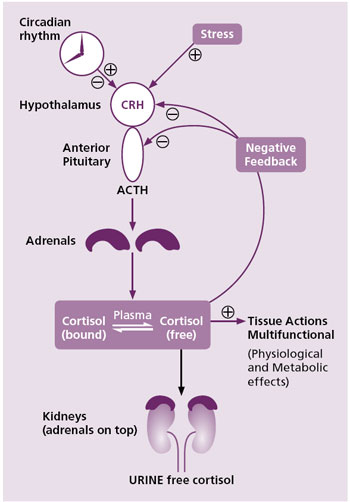
Figure 1. Control and metabolism of cortisol
Mechanisms that initiate the regulation of cortisol release include:
- Circadian rhythm (diurnal variation) which regulates cortisol release throughout the day with the lowest levels late in the evening to around midnight and maximum levels around 08-09 in the morning (Figure 2).
- Homeostasis of cortisol is maintained through a negative feedback mechanism. This can occur at the level of either the hypothalamus or the pituitary as shown in Figure 1.
- Stress, e.g., exercise, also stimulates the release of CRH through neurogenic amines. The negative feedback of cortisol on ACTH can be overridden by stress. Consequently, stress is an important regulator of cortisol in humans.
Irregularities in Cortisol Release (common causes of CS)
Irregularities or dysfunction in cortisol release leading to high cortisol levels (CS) originate from several causes including the following with their percentage occurrences (3, 4):
- pituitary tumor (Cushing’s disease: 65-70%).
- adrenocortical tumors, either benign, malignant or by bilateral adrenal hyperplasia or dysplasia (CS: 15-20%).
- Tumors external of the hypothalamic-pituitary-adrenal axis (ectopic ACTH: 10-15%).
The diagnosis of these irregularities or a deficiency of cortisol as well as clinical dosage adjustment of glucocorticoids require the measurement of serum, salivary or urinary free cortisol concentrations.
Tests for Cushing’s syndrome
Tests commonly recommended for the initial screening and diagnosis of CS include (5):
- 24 hour urine free cortisol (UFC)
- assessment of cortisol circadian rhythm using a late-night serum or salivary cortisol
- low dose dexamethasone (DST) suppression testing
- the dexamethasone-CRH test
When testing salivary or urinary free cortisol, multiple measurements as opposed to a single one are usually required or preferable for a successful diagnosis of cyclical CS.
24 hour Urinary free cortisol (UFC)
Urine free cortisol represents the blood free cortisol that has circulated and is subsequently filtered through the glomerulus into urine during a 24 hour period. It is therefore an integrated measure of free cortisol within a 24 hour period. Thus, collection of urine must be complete during the 24 hour period in order to get an accurate representation of the patients cortisol level (Figure 2). Over collection of urine can give false positive results. This is usually controlled by the simultaneous measurement of urine creatinine.
Creatinine analysis of the 24 hour sample can be used in two important ways:
- to verify the completeness of collection during the 24 hour period and
- to eliminate renal impairment (when the creatinine clearance is less than 60 mL/min) which gives falsely low results.
UFC screens and distinguishes individuals with CS from those without the syndrome, or from those with an intermediate value who require further testing to exclude them from a pseudo-Cushing’s condition.
Sample collection for Cortisol assay.
Since cortisol release of normal individuals is dependent on the diurnal rhythm of the hypothalamic-pituitary-adrenal axis as well as the cortisol circadian variation during the day, it is important to adhere to the sampling protocol in order to prevent potential inaccuracies in cortisol results.
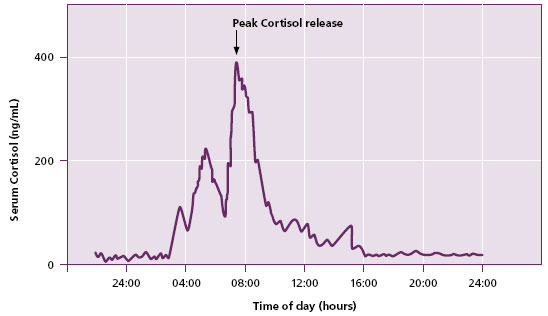
Figure 2. Diurnal cortisol concentration profile of a normal person.
Blood or saliva should be collected at midnight to provide information on cortisol diurnal rhythm. Midnight blood sampling has the disadvantage that patients be admitted to the hospital.
For 24 hour urine collections, 25 mL of 50% acetic acid or 10 g boric acid should be added to the collection vessel at the beginning of collection. Alternatively, urine samples should be refrigerated if no preservative is added during collection. Specifically, the first morning void is discarded; the rest is collected but includes the next morning void. Since the results are based on total volume, it is critical to measure the volume accurately. Only a 20 mL aliquot of the total collection is required to be sent to the laboratory for UFC measurement.
Cortisol assays and laboratory diagnoses
Regardless of the sample type (urine, saliva or serum), the quality and performance of cortisol assay is critical for the proper diagnosis of CS.
Various biochemical methodologies are available for UFC diagnosis of CS (and or pseudo-Cushing’s). They include immunoassays (antibody based) and chromatography (structurally based such as HPLC, GC/MS, and most recently LC/MSMS). Healthcare providers therefore need to be aware of the robustness and quality of results they receive for tests performed on their patients.
Immunoassays for urine free cortisol
Immunoassays for cortisol are designed primarily to measure total serum or plasma cortisol as well as free serum or plasma cortisol. Total cortisol assay helps in clinical evaluation of adrenal disorders whereas free cortisol is useful for the diagnosis of CS. Plasma free cortisol increases as plasma cortisol levels increase. Antisera designed for measuring plasma cortisol are usually adapted to measure urine cortisol as well. Thus, they are generally more specific than urine cortisol due to absence of the type of interfering analytes found in urine (cortisol precursors or metabolites). Various studies have shown that immunoassays for urine free cortisol generally give much higher measured results than chromatographic methods due to the presence in urine of cross-reacting steroid metabolites (naturally occurring or administered whose structures are similar to cortisol) such that antibodies are unable to distinguish them from cortisol, e.g., cortisone, shown below (6).
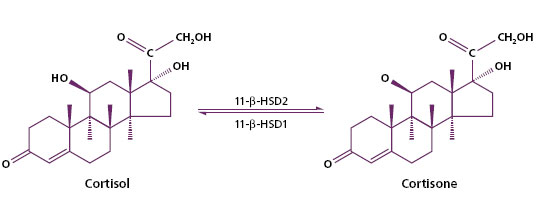
Similar findings have been reported for free salivary cortisol (7).
These metabolites (known and unknown) are responsible for the cross-reactions or interferences associated with many immunoassay kits, thus overestimating the measured cortisol values (Table 1). Cross-reactions ranging from 2-50% have been reported when methodologies such as RIA, Fluorescence polarization, ELISA, and Chemiluminescence (8) have been used for the analysis of cortisol. This is especially true for immunoassays performed without sample pretreatment to remove cross-reactants such as 21-deoxycortisol, corticosterone and prednisolone.
| Immunoassay (μg/24hours) | Chromatography (μg/24hours) | Ref. |
| 44.88 – 332.67 | 6.87 – 62.26 | 9 |
| < 108 | 5.00 – 55.00 | 6 |
| 24 – 108 | 7.96 – 55.20 | 12 |
| 176 – 1849 | 10.86 – 52.49 | 11 |
Table 1. Urine free cortisol levels by immunoassays compared to chromatographic methods.
Chromatography
Chromatographic methodologies such as liquid chromatography with UV detection (HPLC-UV) and gas chromatography mass spectroscopy (GC-MS) are generally less affected by cross-reactions than immunoassays. Chromatographic methods are able to remove/separate a significant amount of the interfering substances thus giving a more accurate assessment of cortisol measurements than immunoassays. However HPLC-UV measurement of cortisol can suffer interferences when patients are on medications such as carbamazepin (9) or digoxin (10) and fenofibrate.
No cross reactions (<1%) are associated with UFC by liquid chromatography tandem mass spectroscopy (LC-MSMS). In some cases, sample pretreatment removes nearly all interfering metabolites from the sample before application to the LC-MSMS. Additionally, LC-MSMS provides a multi-analyte profiling from a single run separating cortisol from any metabolites that might be present in the sample.
Figure 3 illustrates this point where a urine sample after a simple liquid-liquid extraction in our laboratory separated cortisol (at time = 3.30 minutes) from cortisone (at time = 2.74) (a). The detector further confirms the identity of the analytes by their characteristic transitions (b). Thus, the true value of free cortisol is measured and reported which is much lower than results obtained by immunoassay as shown in Table 1.
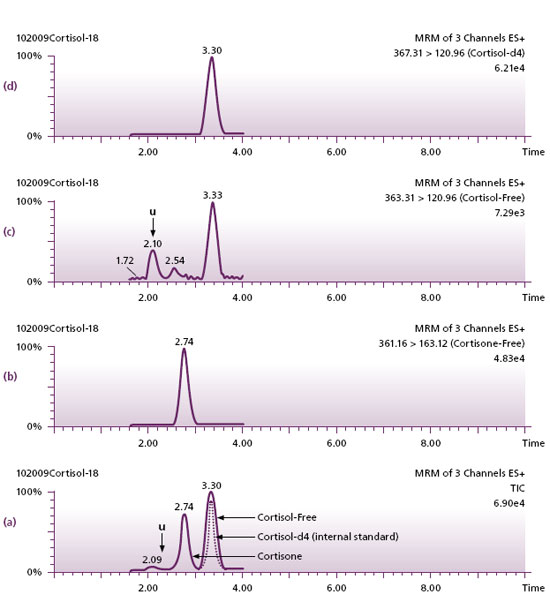
Figure 3. (a) Chromatography of cortisol showing the separation from cortisone and another unidentified analyte “U”. (b, c, d) tandem mass spectrometric confirmation of the identities of cortisone and cortisol by way of their unique masses. Cortisol-Free and cortisol-d4 not separated by chromatography (a), has been separated by the mass spectrometer (c and d).
Conclusion
Although the immunoassay screening methods recommended by the Endocrine Society (13) show comparable clinical sensitivity with LC-MSMS, since LC-MSMS demonstrates the best specificity (>90%), Warde Medical Laboratory recommends this technique in screening for CS.
References
- Deborah M. and Cecilia AL. Emerging Role for Tandem Mass Spectrometry in Detecting Congenital Adrenal Hyperplasia. Clin Chem 2004 50: 467-468.
- NIH Publication No. 08-3007, July 2008.
- Orth DN. Cushing's syndrome. N Engl J Med 1995; 332:791-803.
- Newell-Price, J. Trainer, P. Besser, GM. Grossman, AB. The diagnosis and differential diagnosis of Cushing's syndrome and pseudo-Cushing's states. Endcr Rev 1998; 19:647-672.
- Analdi G, Angel A. Atkinson AB, Bertagna X, Cavagnini F, Chrousos GP. Diagnosis and complication of Cushing's syndrome: a consensus statement. J. Clin Endocrinology Metab. 2003; 88:5593-5602.
- Taylor RL, Machacek D, Singh RJ. Validation of a high-throughput liquid chromatography-tandem mass spectrometry method for urinary cortisol and cortisone. Clin. Chem. 2002;48:1511-1519.
- Baid SK, Sinaii N, Wade M, Rubino D, Nieman LK. Radioimmunoassay and tandem mass spectrometry measurement of bedtime salivary cortisol levels: a comparison of assays to establish hypercortisolism. J Clin Endocrinol Metab. 2007; 92:3102-3107.
- Horie H, Kidowake T, Koyoama Y. Specificity assessment of immunoassay kits for determination of urinary free cortisol concentrations. Clin. Chem. Acta 2007; 378:66-70.
- Ching SY, Lim EM, Beilby J, Urine free cortisol analysis by automated immunoassay and HPLC for the investigation of Cushing's syndrome. Ann Clin Biochem. 2006; 43: 402-407.
- Findling JW, Raff H. Newer diagnostic techniques and problems in Cushing's disease. Endocrinology Metab Clin North Am 1999; 28:191-210.
- Turpeinen U, Markkenen H, Valimaki M, Stenman U. Determination of urinary free cortisol by HPLC. Clin. Chem. 1997; 43:1386-1391.
- Lin CL, Wu TJ, Machacek DA, Jiang NA, and Kao PC. Urinary free cortisol and cortisone determined by high performance liquid Chromatography in the diagnosis of Cushing's syndrome. J Clin Endocrinol Metab. 1997; 82:151-155.
- Gilbert R and Lim EM. The diagnosis of Cushing's syndrome: An endocrine society clinical practice guideline. Clin. Biochem Rev. 2008; 29:103-106.

