Update: Flow Cytometric Immunophenotyping of Hematolymphoid Neoplasia
 Introduction
Introduction
Flow cytometric (FCM) immunophenotyping continues to have an ever expanding role in the diagnosis and
prognostication of leukemias, lymphomas and related hematolymphoid neoplasias 1–4. However, at the same time, pathologists and laboratorians are faced with increasing demands for both timely reporting of biopsy results and control of the cost of testing. This update reviews indications and approaches for FCM immunophenotyping of leukemia, lymphoma and myelodysplasia, and touches upon some of the controversies. As part of this, recent changes in Warde Medical Laboratory’s flow services for leukemia / lymphoma phenotyping are discussed. Lastly, illustrative cases are presented.
Your — The Client’s — Role in Pre Analytic Processing
This is really the most important phase of the process. As part of this, it is imperative that the Client laboratory do a timely review of the morphology, along with any available laboratory and clinical features of the case. This will allow for a
- rapid “go / no-go” decision to perform phenotyping, and
- the selection of the flow panel(s) that will yield clinically and diagnostically relevant information —
- all done in a time period that allows the specimen to be delivered in optimal condition.
PROCESS / NO PROCESS TRIAGE BY THE CLIENT
1. Clinical and Laboratory History
The physician must decide whether immunophenotyping would be medically useful, and if so, what panel of antibodies should be evaluated. Not all hematolymphoid neoplasias (HLN) are reasonably in the differential diagnosis of all tissue biopsies.
“Medical Usefulness” may be initially defined by:
- Relevant laboratory data (CBC & differential; serum / urine protein electrophoresis) and (ideally);
- clinical information, including medical urgency (e.g. mediastinal syndrome; severe cytopenias and DIC).
Clinical Case 1: History
A 47 year old male presents with a one month history of difficulty of swallowing. The physician’s physical exam reveals asymmetrically enlarged tonsils, with the left being significantly more enlarged than the other. No enlarged local or distant lymph nodes are found. Concomitant blood studies reveal a mild normochromic anemia with an otherwise normal CBC / differential and serum biochemical profiles.
Clinical Case 2: History
A 54 year old female presents with history of fatigue. The physical exam is unremarkable. Laboratory studies reveal only mild persistent normochromic anemia associated with a modest increase in the calculated serum globulin fraction. A serum protein electrophoresis and immunofixation reveals a small (0.7 gm/dL) IgA Lambda monoclonal gammopathy.
Clinical Case 3: History
A previously healthy 34 year old female present with malaise and easy bruising. The physical exam is otherwise unremarkable. The CBC reveals profound pancytopenia.
2. Sample Type & Morphology:
The type of the solid tissue biopsy and incidence of HLN allow for effective triage decisions 5. Excisional biopsies of solid tissue can usually be diagnosed with morphologic exam combined, as necessary, with fixed tissue immunohistochemistry and/or T or B gene clonality assays. This, and the stability of cell suspensions confidently allows for a 36 to 48 hour “window” in which morphologic and clinical evaluation can be performed without compromising overall diagnostic capabilities.
On the contrary, fine needle aspirate (FNA) biopsies of a suspected HLN tumor should have a restricted flow panel run, given the frequent absence of histology and scanty nature of the FNA. However, if at all possible, a rapid review of the FNA cytology should be performed to see if the morphology will obviate the need for flow (eg, metastatic carcinoma is present). As most solid tissue HLNs in North America are mature B lymphoid or Hodgkin’s lymphomas 5, a flow panel directed at mature B lymphoid neoplasias with CD5, CD10, CD11c, CD19, CD20, CD23, CD38 and CD45 differentiation and immunoglobulin light chian clonality markers would be an appropriate “blind” panel to order with such FNAs.
A liquid hematology sample (eg, blood, marrow, CSF) can be rapidly stained for cytologic exam, and significant laboratory information is often easily obtained (CBC; differential, protein electrophoresis). These will often allow for rapid triage and, if indicated, selection of a focused flow panel. Any of the three typical anticoagulants (acid citrate dextrose (ACD), ethylenediaminetetraacetic acid (EDTA) or sodium heparin) will maintain marrow or blood cell viability and antigenicity for 48 hours at room temperature.
Sample viability can be further extended to up to 96 hours by refrigeration and/or 1:1 dilution with cell media (e.g. RPMI 1640, McCoy’s). A CSF sample is a special exception, where immediate dilution (1:1 in cell media containing 5% bovine or new born calf albumin is necessary to adequately preserve the cells. Other fluid samples (e.g. pleural; peritoneal) should be stored in sterile containers. If transport / storage time is greater than 24 hours, then refrigerated temperatures are preferred, with or without dilution with cell media.
Peripheral blood samples have a more limited spectrum of cells. Thus, a more restricted flow panel is often a very effective approach, typically directed at mature B lymphoid neoplasms. Marrow samples, on the other hand, are more complex, and any abnormal cells may be in very low concentration. As a result, flow sample panels run for marrow are often larger, particularly if the clinical and morphologic differential is broad (e.g. cytopenias — rule out acute leukemia, myelodysplasia, clonal B cells, T LGL).
Low cellularity does not exclude flow analysis of either solid or liquid samples. A single tube with a minimum of 3- to 4-color reagents is often able to answer basic clinically relevant questions (e.g. B cell clonality) with low cellularity. The Warde Medical Laboratory flow laboratory will always try to analyze a low cellularity sample. In such cases, and, if need be, in consultation with the referring clinician or laboratory, the flow laboratory may choose a more concise panel of markers to answer the most medically significant question(s).
Clinical Case 1: Sample Type, Timing & Rapid Morphologic Exam
On Friday afternoon, excisional biopsies of both the right and left tonsils are received. Touch imprints are made and rapidly Wright-stained, revealing a relatively monomorphic intermediate size population of mature lymphoid cells. Given that histologic exam will be done three days later (Monday) and increased probability of lymphoma (clinical, laboratory and cytologic abnormalities seen on rapid review), a decision is made to perform flow cytometry.
Clinical Case 2: Sample Type, Timing & Rapid Morphologic Exam
An aspiration and core marrow biopsy are received. A single aspirate smear is Wright-stained, revealing a minimally increased fraction (< 10%) of cytologically normal plasma cells. The pathologist wishes to unambiguously define the presence or absence of a plasma cell dyscrasia (monoclonal proliferation). Therefore, flow cytometry is ordered.
Clinical Case 3: Sample Type, Timing & Rapid Morphologic Exam
The marrow aspiration cytology reveals a significantly increased fraction of large undifferentiated blasts (~ 30%). Based upon the patient’s age and these cytologic findings, flow cytometry is ordered to confirm the general diagnosis of acute leukemia, and to establish lineage / immunophenotypic “footprint” for evaluation of relapsed or minimal residual disease.
PANEL SELECTION
There is no body of evidence-based medicine that supports the routine use of broad-spectrum flow panels to improve diagnostic accuracy and/or patient outcomes, as compared to more focused panels based upon morphologic and/or clinical triage.
Thus, the Client / Reference Laboratory “team” approach is utilized by Warde Medical Laboratory. For optimal medical quality, the client should provide information that allows our team to perform an effective triage based upon:
- sample type / source,
- basic patient demographics (eg, adult vs. pediatric) and
- laboratory data (eg, CBC, protein electrophoresis) and, optimally, rapid cytologic screen.
In our experience, one or, at most, two of the standard Warde Medical Laboratory flow panels will suffice for diagnostic evaluation in virtually all instances (see table 1). Indeed, if “all” or “no” panels are selected on the flow order form that we receive, the processing will be halted until the client defines a concise set of markers, or, in consultation with a Warde Medical Laboratory flow pathologist, clearly defines a need for large multi panel analysis.
1. B Cell & Hairy Cell Panels
The B Cell Panel is ideal for diagnostic evaluations of lymph nodes in adults where T cell neoplasia is not in the differential diagnosis (see below). This is particularly true for FNA biopsies, where the additional differentiation markers (CD10, 20, 23) may offset the absence of histology in allowing a more refined diagnosis. The panel is also effective in staging marrow evaluations of diagnosed CD10+ mature B NHLs, although the smaller B cell clonality panel will often suffice, particular for the CD10 negative B cell neoplasias. If cytologic exam or clinical history indicates the possibility of Hairy Cell Leukemia, then the corresponding panel should be ordered. Interestingly, if a lymphoplasmacytic lymphoma, IgM monoclonal gammopathy and/or Waldenstrom’s macroglobulinemia are in the differential diagnosis, then assessment of the B lymphoid compartment of the marrow should be performed as part of the diagnostic evaluation, using the B Cell panel 6, 7.
2. B Cell Clonality (Short B Cell) Panel
Typically, this is used for staging or relapse assessment of a previous diagnosed mature B cell neoplasm in solid or liquid samples. As mentioned above, when a marrow sample is being assessed for staging or relapse of a mature CD10+ B lymphoid neoplasm, the more extensive mature B cell panel allows for greater clarity is defining hematogones vs. neoplastic cells.
3. Plasma Cell Panel
The diagnostic criteria for myeloma do not require flow analysis 8–10. In the large majority of cases, serum & urine protein electrophoresis combined with quantitative cytologic and histology exam of the native marrow aspirate and biopsy will suffice for diagnosis along with relevant clinical data.
Further, as virtually all myeloma therapy is not expected to be curative, evaluations for MRD in myeloma by flow are not standard of practice. The best indication for plasma cell analysis in the setting of myeloma may be to confirm the pathologist’s cytologic impression of residual neoplastic plasma cells. A related application may be with FNAs of solid tissues or non-marrow fluid sample plasmacytosis, where flow can clearly define plasma cell clonality.
In contrast, for IgG or IgA monoclonal gammopathies of uncertain significance (MGUS) and smoldering myeloma, the presence (> 5% of plasma cells) of atypical (clonal) CD19 Negative / CD56± plasma cells is associated with a higher risk of progression to clinically overt myeloma 11. However, alternative, non-flow prognostic algorithms have also been put forward for prognostication of smoldering myeloma, which may be more attractive as they do not require marrow samples 9.
One last comment is that flow plasma cell analysis should not be used for quantitative purposes (eg, confirming cytologic quantitation). Plasma cells are selectively lost during staining processing for flow. This is unavoidable, and reflect the relative fragility of both benign and neoplastic plasma cells vis a vis other marrow cells.
4. Mature T cell Panel
Mature T lymphoid neoplasms are uncommon-to-rare in North American populations 5, 12. Those which are most amenable to flow analysis usually have one or more distinct features that will flag a case as suspect for T lineage.
- Atypical cytology — large granular lymphocytosis with or without neutropenia; cerebriform or markedly pleomorphic lymphoid cytology consistent with Sézary or Adult T Cell neoplasms.
- Clinical history — of a T cell lymphoma, extranodal presentation of a lymphoproliferation, an unexpected clinically aggressive lymphoproliferation (eg, “CLL” presenting with 100,000+ lymphs per uL), and/or otherwise unexplained hypercalcemia.
Currently, the Warde Medical Laboratory flow laboratory does not perform TCR Vbeta class analysis. This is a complex 24 marker study to evaluate for T cell clonality 13. It is recommended that clients wishing to confirm the monoclonality of T cell proliferations utilize PCR assays for TCR gamma gene clonality. It is less expensive, better validated and more widely applicable to the range of tissue types and fixation.
While the flow analysis can confirm T lineage of a lymphoproliferation and identify abnormalities suggestive of T neoplasia, flow analysis is not very specific for individual categories of T leukemia / lymphoma. Exceptions are T large granular lymphoid leukemia and T lymphoblastic lymphoma / leukemia. As a corollary, extensive clinical, morphologic and other laboratory data are often required for the precise diagnosis of most T cell neoplasias 14.
5. Acute Leukemia
The selection of this panel is typically fairly straightforward. If morphologic exam of liquid or, less often, solid tissue samples reveals an excess of blasts, then the acute leukemia panel should be run to confirm the abnormal excess or presence of the blasts, and define clinically significant lineages (T vs. B lineage ALLs vs. AML). The basic acute leukemia panel will accomplish this in the vast majority of cases. In unusual instances, the Warde Medical Laboratory pathologist and/or the client may add cytoplasmic markers to clarify lineage assignment (TdT, myeloperoxidase/ MPO; CD3, CD79a).
In the last 7 years, several studies have demonstrated that there are reproducible immunophenotypic abnormalities seen in myelodysplasia 15–19. The best characterized include excess of myeloblasts and immunophenotypic (IP) abnormalities of myeloblasts, as well as flow light scatter and IP abnormalities of more mature myeloid precursors and monocytes. Thus, in the absence of an acute leukemia, finding such changes can suggest the possibility of MDS. Most of these can be detected by the basic Acute Leukemia panel.
Unfortunately, these changes are not diagnostic, and have not yet been formally included in modern formal diagnostic classification of MDS 20. Thus, it is not recommended that the initial exam of a marrow for MDS include flow. However, if the initial workup is non-diagnostic, then when a repeat marrow is performed, flow analysis may assist in clarification. There are several reasons for this reserved role for FCM in the workup of MDS. Beyond the absence of formal criteria in WHO classifications, there is the issue of lack of standardization of flow cytometer set up, and antibody and fluorochrome combinations. Lastly, in any given non-MDS case, a few of the changes seen with MDS may be present (eg, CD56 expression on myeloid precursors after chemotherapy). In other words, there is a lack of clinical specificity when only a few IP abnormalities are present.
Clinical Case 1: Panel Selection
Based upon the age of the patient (adult), location of “tumor” (Tonsils; extranodal) and no evidence of lymphadenopathy or lymphocytosis, a panel directed at mature B cell neoplasms is ordered.
Clinical Case 2: Panel Selection
Based upon the age of the patient (adult), location (marrow), evidence of monoclonal gammopathy associated with borderline elevation of cytologically normal plasma cells, a panel directed defining plasma cell clonality is ordered.
Clinical Case 3: Panel Selection
Based upon the age of the patient (adult), history (pancytopenia), sample rapid cytology (increased blasts) and location of sample (bone marrow), a panel directed at the major types of acute leukemias is ordered.
WARDE MEDICAL LABORATORY REPORTING OF RESULTS
Interpretation
The Warde Medical Laboratory flow laboratory’s pathologist will review the available cytology and any clinical history along with the patterns and quantitative analysis of immunophenotypes and light scatter. If any atypical population is detected, the antigen profile will be listed, and possible / favored differential diagnoses put forth. Lastly, suggestions will be given if any ancillary testing may clarify the differential (eg, cytogenetic FISH exams, T or B gene clonality assays).
A well established caveat with flow analysis is that the client must correlate these results with their biopsy histology or native aspirate cytology and any clinical information the client would usually require for morphologic diagnosis. This will guard against any FCM false negative results due to unavoidable loss of neoplastic cells during processing (eg, some plasma cell dyscrasias; large cell NHLs), or overinterpretation of a “reactive” monoclonal being interpreted as neoplastic 21, 22. Lastly, with few exceptions, most neoplastic FCM profiles are not specific for a particular type of leukemia or lymphoma — the final specific diagnosis will require at least morphology and, not infrequently, molecular / genetic analysis.
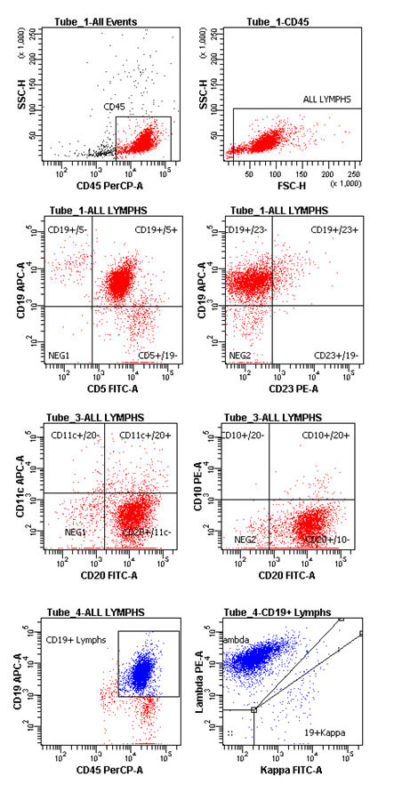
Clinical Case 1: Flow Panel Profile
There is a predominant monoclonal (Lambda) B lymphoproliferation (Tube #4). Virtually all the B cells (CD19+ or CD20+) are CD5+ (Tube #1), while lacking expression of CD10, CD11c (Tube #3) or CD23 (Tube #1). The intensities of CD20 (tube #3) and Lambda Light chain (tube #4) are equivalent to normal B cells, while the intensity of CD19 is mildly abnormally dimmer than normal B cells (CD19+/5- cells; Tube #1).
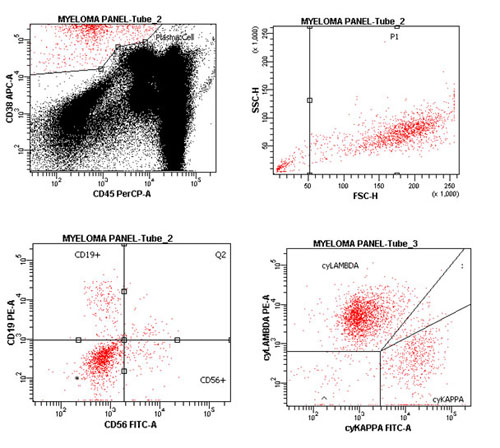
Clinical Case 2: Flow Panel Profile
There is a predominant monoclonal (Lambda) plasma cell dyscrasia. Virtually all the plasma cells have a membrane profile of CD19 Negative / CD56+, and are cytoplasmic Lambda+/Kappa Neg.
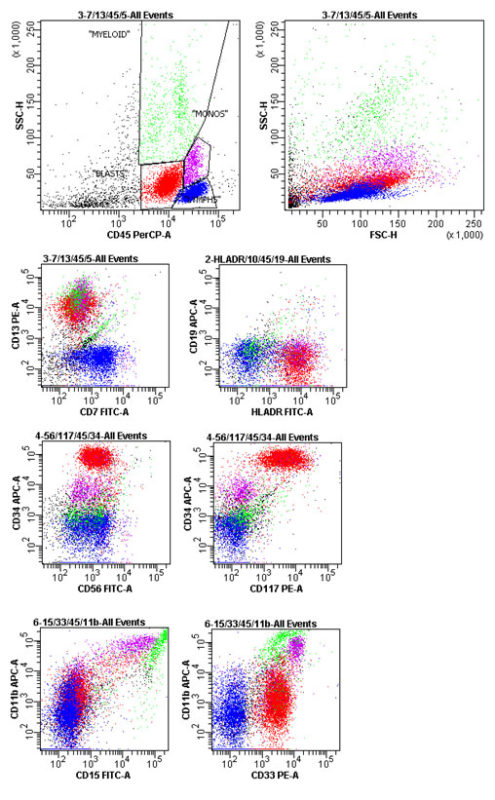
Clinical Case 3: Flow Panel Profile
Approximately 40% of the cells are myeloblasts, with a composite antigen profile of: CD7 Neg., 11b+ (partial; dim), 13+, 15 Neg., 19 Neg., 33+, 34+, 56+ (dim), 117+ & Dr+.
Reporting — Content
The report will begin with a listing of the sample type provided by the client, and the antigens used in analysis. Warde Medical Laboratory’s flow laboratory has ceased listing the percentage of reactive cells, which is fully in line with recent international recommendations 1, 23, and reflects that it is the laboratorian’s interpretation of the composite FCM patterns that provides the diagnostic power — not just individual antigen reactivity percentages. The latter are still provided in the table below each dot plot.
Several of our clients prefer to do their own interpretations. In this instance, a standard comment is added to our Warde Medical Laboratory flow report to indicate this, and the report and the color dot plots are sent to the client via a secure e-mail server. For other clients, a full interpretation will be provided by the Warde FCM pathologists. The will include the concise cytologic features seen on our Wright-stained cytospin or blood smear preps.
If an abnormal population is detected, then the composite antigen profile will be given, along with any abnormal intensities or distribution of antigen intensities that may assist in the evaluation. A differential diagnosis will be attempted for such populations. Any follow up studies that will clarify less definite flow diagnoses should be concisely suggested. After the electronic signature by the interpreting pathologist, a list of the monoclonal antibodies used will be reported. Lastly, a standard comment required by the FDA for assays using ASR reagents will be added.
Reporting — Turnaround Time
Once the sample is received by Warde Medical Laboratory and a is panel ordered, most cases should be stained, analyzed, interpreted and reported within 1-2 working days. Since the flow analysis is routinely performed 6 days per week (Monday through Saturday, extended day shift), we expect that 90% of the cases will be reported by 5pm the day following sample receipt.
Reports are issued on the day following receipt on Sunday through Thursday. Samples received on Friday are triaged for final analysis and interpretation on Saturday — if it is an acute leukemia panel or a request for a “stat” analysis has been made, then the interpretation will be provided on that Saturday. Otherwise, all samples with other panels will be processed, stained and data acquired on Saturday. The final technical analysis of the data and subsequent professional interpretation will be provided on Monday morning.
If a “stat” analysis is desired on any day, please contact the Warde Medical Laboratory client services and ask to immediately speak to the Warde Medical Laboratory Flow Cytometry Pathologist, Dr. Keren, or, myself (Dr. Carey).
Clinical Case 1: Reports (antibody list & FDA comment not shown). Interpretation:
Tonsil, Left: Monoclonal CD5+ B Cell Neoplasm, Fully Consistent with Mantle Cell Lymphoma.
Comments
The bright CD45+ cells with small-and-large lymphoid light scatter are analyzed. These are predominantly monoclonal B cells (88% of lymphoid cells), with a composite antigen profile of: CD19+ (mildly abnormally dimmer), 20+ (normally bright), 5+, 10 Neg., 11c, Neg., 23 Neg., 45+ (normally bright) and Lambda+ (normally bright).
The profile is fully consistent with Mantle Cell NHL. Confirmation should be established with routine histology, along with bcl1 immunoreactivity and/or positive FISH (fluorescence in situ hybridization) cytogenetic exam for t(11;14) CCND1-IgH fusion signal.
Pathologist:
John L. Carey, MD
Clinical Case 2: Reports (antibody list & FDA comment not shown). Interpretation:
Marrow, Aspirate: Monoclonal CD19 Neg./CD56+ Plasma Cell Dyscrasia.
Comments
The bright CD38+ cells with bright-to-absent CD45 expression and plasma cell light scatter are analyzed. These are predominantly monoclonal plasma cells (90% of plasma cells), with a composite antigen profile of: CD19 Neg., 38+ (bright), 45 Neg., 56+ and cyto. Lambda+.
The profile is fully consistent with a plasma cell dyscrasia. The final classification of this neoplasm will require correlation with the cytologic evaluation of the native marrow aspirate, along with serum / urine protein electrophoresis.
Pathologist:
Daniel Mais, MD
Clinical Case 3: Reports (antibody list & FDA comment not shown). Interpretation:
Marrow, Aspirate: Fully Consistent with Acute Myelogeneous Leukemia.
Comments
The dimmer CD45+ cells with blast light scatter are analyzed. These are virtually all atypical myeloblasts, comprising 40% of the flow sample, and have a composite antigen profile of:
CD11b+ (partial; dim), 13+, 15 Neg., 33+, 34+, 56+ (dim), 117+ & Dr+.
The profile is fully consistent with an AML. Please correlate with the morphology of the native smear and biopsy, cytogenetics and all other rele vant clinical and laboratory data.
Pathologist:
Ann Alpern, MD
Table 1. Warde Medical Laboratory Flow Immunophenotyping Panels
for Hematolymphoid Neoplasia.
| Panel Name (order Code) | Tube # | Antigens (4 Color) |
| Mature B Cell (BCELL) | 1 2 3 4 5 (leb option) | CD5/ CD23/ CD45/ CD19 CD20/ CD38/ CD45/ CD3 CD20/ CD10/ CD45/ CD11c Kappa/ Lambda/ CD45/ CD19 Control/ Control/ CD45/ Control |
| B Cell Clonality (SHORTBCELL) | 1 2 3 (lab option) | CD5/ CD23/ CD45/ CD19 Kappa/ Lambda/ CD45/ CD19 Control/ Control/ CD45/ Control |
| Hairy Cell Leukemia (HAIRYCELL) | Mature B Cell + 6 | CD103/ CD25/ CD45/ CD22 |
| Mature T Cell (TCELL) | 1 2 3 4 5 (lab option) 6 (client option) | CD7/ CD4/ CD45/ CD3 CD57/ CD8/ CD45/ CD3 CD56/ CD16/ CD45/ CD5 CD2/ CD25/ CD45 Control/ Control/ CD45/ Control TCRab/ TCRgd/ CD45/ CD3 |
| Plasma Cell (MYELOMA) | 1 2 3 | CD56/ CD19/ CD45/ CD38 Control/ Control/ CD45/ CD38 Cyto. Kappa/ Cyto. Lambda/ CD45/ CD38 |
| Acute Leukemia (ACUTE) | 1 2 3 4 5 6 7 (client/lab option) 8 (client/lab option) 9 (lab option) | HLA Dr/ CD10/ CD45/ CD19 CD7/ CD13/ CD45/ CD5 CD56/ CD117/ CD45/ CD34 CD61/ CD235a/ CD45/ CD14 CD15/ CD33/ CD45/ CD11b Control/ Control/ CD45/ Control Cyto. MPO/ cyto. CD79a/ CD45/ cyto. CD3 Cyto. TdT/ cyto. CD79a/ CD45/ cyto. CD3 Cyto. Control/ Cyto. Control/ CD45/ cyto. Control |
Clinical Case 1: Key Flow Cytometric Dot Plots
Clinical Case 2: Key Flow Cytometric Dot Plots
Clinical Case 3: Key Flow Cytometric Dot Plots
References
- Stetler-Stevenson, M., E. Ahmad, D. Barnett, et al., eds. Clinical Flow Cytometric Analysis of Neoplastic Hematolymphoid Cells: Approved Guidelines. Second Edition — CLSI document H43-A2 ed. Clinical and Laboratory Standards Institute. 2007, Clinical and Laboratory Standards Institute, 940 West Valley Road, Suite 1400, Wayne, Pennsylvania 19087-1898 USA: Wayne, Pennsylvania 19087-1898.
- Davis, B.H., J.T. Holden, M.C. Bene, et al., 2006 Bethesda International Consensus recommendations on the flow cytometric immunophenotypic analysis of hematolymphoid neoplasia: Medical indications. Cytometry Part B: Clinical Cytometry, 2007. 72B(S1): p. S5-S13.
- Stetler-Stevenson, M. and J.A. Schrager, Flow Cytometric Analysis in the Diagnosis and Prognosis of Lymphoma and Chronic Leukemias, in Flow Cytometry in Clinical Diagnosis, 4th Edition, J.L. Carey, J.P. McCoy, and D.F. Keren, Editors. 2007, American Society of Clinical Pathology Press: Chicago, IL. p. 129-167.
- Kroft, S.H. and N.J. Karandikar, Flow Cytometric Analysis of Acute Leukemias, Myelodysplastic Syndromes, and Myeloproliferative Disorders, in Flow Cytometry in Clinical Diagnosis, 4th Edition, J.L. Carey, J.P. McCoy, and D.F. Keren, Editors. 2007, American Society of Clinical Pathology Press: Chicago, IL. p. 168-214.
- Siebert, J.D., D.A. Mulvaney, K.L. Potter, et al., Relative Frequencies and Sites of Presentation of Lymphoid Neoplasms in a Community Hospital According to the Revised European-American Classification. American Journal of Clinical Pathology, 1999. 111(3).
- Owen, R.G., S.L. Barrans, S.J. Richards, et al., Waldenstrom Macroglobulinemia: Development of Diagnostic Criteria and Identification of Prognostic Factors. American Journal of Clinical Pathology, 2001. 116: p. 420-428.
- Dimopoulos, M.A., R.A. Kyle, A. Anagnostopoulos, et al., Diagnosis and Management of Waldenstrom’s Macroglobulinemia. Journal of Clinical Oncology, 2005. 23(7): p. 1564-1577.
- Kyle, R.A. and a.t.I.M.W. Group, Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the Internation Myeloma Working Group. British Journal of Hæmatology, 2003. 121: p. 745-757.
- Kyle, R.A., E.D. Remstein, T.M. Therneau, et al., Clinical Course and Prognosis of Smoldering (Asymptomatic) Multiple Myeloma. N Engl J Med, 2007. 356(25): p. 2582-2590.
- Anderson, K.C., R.A. Kyle, S.V. Rajkumar, et al., Clinically relevant end points and new drug approvals for myeloma. Leukemia, 2007. 22(2): p. 231-239.
- Perez-Persona, E., M.-B. Vidriales, G. Mateo, et al., New criteria to identify risk of progression in monoclonal gammopathy of uncertain significance and smoldering multiple myeloma based on multiparameter flow cytometry analysis of bone marrow plasma cells. Blood, 2007. 110(7): p. 2586-2592.
- Jaffe, E.S., L. Krenacs, and M. Raffeld, Classification of T-cell and NK-cell Neoplasms Based on the REAL Classification. Annals of Oncology, 1997. 8(Suppl 2): p. S17-S24.
- Lima, M., J. Almeda, A.H. Santos, et al., Immunophenotypic Analysis of the TCR-Vb Repertoire in 98 Persistent Expansions of CD3+ / TCRab+ Large Granular Lymphocytes. American Journal of Pathology, 2001. 159: p. 1861-1869.
- Jaffe, E.S., L. Krenacs, and M. Raffeld, Classificatioin of Cytotoxic T-Cell and natural Killer Cell Lymphomas. Seminars in Hematology, 2003. 40(3): p. 175-184.
- Stetler-Stevenson, M., D.C. Arthur, N. Jabbour, et al., Diagnostic utility of flow cytometric immunophenotyping in myelodysplastic syndrome. Blood, 2001. 98: p. 379-387.
- 16. Kussick, S., J.R. Fromm, A. Rossini, et al., Four-color flow cytometry show strong concordance with bone marrow morphology and cytogenetics in the evaluation for myelodysplasia. American Journal of Clinical Pathology, 2005. 124: p. 170-181.
- Malcovati, I., M.G. Della Porta, M. Lunghi, et al., Flow cytometry evaluation of erythroid and myeloid dysplasia in patients with myelodysplastic syndrome. Leukemia, 2005. 19: p. 776-783.
- Matsui, W.H., R.A. Brodsky, B.D. Smith, et al., Quantitative analysis of bone marrow CD34 cells in aplastic anemia and hypoplastic myelodysplastic syndromes. Leukemia, 2006. 20: p. 458-462.
- Xu, Y., R.W. McKenna, N.J. Karandikar, et al., Flow cytometric analysis of monocytes as a tool for distinguishing chronic myelomonocytic leukemia from reactive monocytosis. American Journal of Clinical Pathology, 2005. 124: p. 798-806.
- Bennett, J.M., A comparative review of classification systems in myelodysplastic syndromes (MDS). Semin Oncol, 2005. 32: p. S3-S10.
- Kussick, S., M. Kalnoski, R. Braziel, et al., Prominent Clonal B cell Populations Identified by Flow Cytometry and Histologically Reactive Lymphoid Populations. American Journal of Clinical Pathology, 2004. 121: p. 464-472.
- Rawston, A.C., M.J. Green, A. Kuzmicki, et al., Monoclonal B lymphocytes with the characteristics of “indolent” chronic lymphocytic leukemia are present in 3.5% of adults with normal blood counts. Blood, 2002. 100: p. 635-639.
- Brent L. Wood, et al., 2006 Bethesda International Consensus recommendations on the immunophenotypic analysis of hematolymphoid neoplasia by flow cytometry: Optimal reagents and reporting for the flow cytometric diagnosis of hematopoietic neoplasia. Cytometry Part B: Clinical Cytometry, 2007. 72B(S1): p. S14-S22.
 Cover art from Flow Cytometry in Clinical
Diagnosis (4th Edition) provided courtesy of the publisher,
The American Society for Clinical Pathology
Cover art from Flow Cytometry in Clinical
Diagnosis (4th Edition) provided courtesy of the publisher,
The American Society for Clinical Pathology 
