Updated TB Testing by QFT-Plus
March 2021
Overview
On March 23rd, Warde Medical Laboratory is upgrading to a new assay platform for diagnosing tuberculosis (TB) infection. This change will not affect patients – the new platform uses the same specialized 1 mL blood collection tubes (BCTs). Providers will notice two minor changes:
1) A new ordering test code (QFTBI)
2) A new requirement to affix a tube-specific label to each of the four BCTs.
Changes in the QFT-Plus Procedure
Beginning on March 23rd, Warde will migrate the QFT®-Plus IGRA to the Diasorin LIAISON® XL. The LIAISON® XL will quantitate Interferon Gamma (IFNγ) by using chemiluminescence (as opposed to a traditional sandwich ELISA), which will allow for a streamlined workflow and a narrower non-reportable range.
On March 23rd; Clients should follow the Quick Guide attached for labeling tubes
Principle of the QFT-Plus Assay
Tuberculosis (TB) is caused by multiple Mycobacterium species referred to as the Mycobacterium tuberculosis complex (MTBC). Interferon Gamma Release Assays (IGRA) are the fastest and most reliable method for detecting MTBC infection. Warde provides high-quality IGRA testing through Qiagen’s QuantiFERON®-TB Gold Plus (QFT®-Plus) IVD test. The QFT®-Plus assay works by incubating patient blood in BCTs containing peptide antigens. The peptides simulate MTBCinfection causing the release of IFNγ from CD4+ and CD8+ T cells that recognize these organisms. The detection of IFNγ in response to these peptides indicates possible MTBC infection.
QFT-Plus Blood Sample Requirements
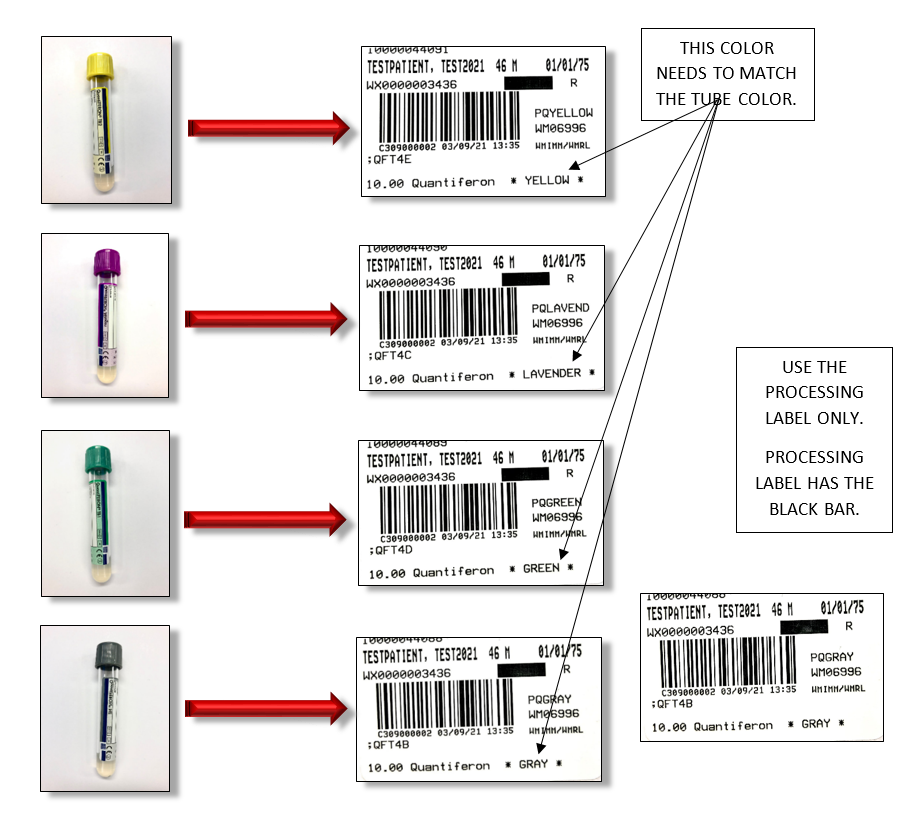
Proper specimen collection and incubation is critical for reliable QFT®-Plus test results. 1 mL of blood is collected into each of the four QFT®-Plus BCTs (a black mark indicates the correct fill volume). The correct label must be affixed to each tube (as shown in the attached labeling guide). . Incorrect labeling may result in a delay or cancellation since the control tubes could fail testing. BCTs must be incubated at 37°C for 16-24 hr and centrifuged prior to shipping. Contact Warde Client Services for questions regarding specimen handling or preparation. The test manufacturer can help eliminate problems in this workflow if issues arise. Testing is performed according to CDC recommendations on patients >5 years of age.
QUANTIFERON – TB PLUS –
Labeling Tubes – Quick Guide
Affix the label with color designation identical to the color of the blood collection tube.
(Color of the tube is indicated on the lower right hand side of the label)
Please see the example below of how to label each tube with the correct COLOR LABEL.
ERRONEOUS RESULTS WILL OCCUR IF THE WARDE SOFT LABEL INDICATING THE COLOR OF THE TUBE IS NOT MATCHED WITH THE CORRESPONDING COLORED TUBE.