Virology Testing Update
Laboratory detection of viral infections has changed significantly in the past 40 years. Animal and egg inoculations, which were routinely used in the 1960s and 1970s, have been replaced by cell culture procedures, enzyme immunoassays (EIA), direct fluorescent assay (DFA) tests, and nucleic acid procedures.
The driving forces behind these changes were the availability of new antiviral therapies, changing third party reimbursement rates, and demands for shorter, clinically relevant, turnaround times. In response to these medical needs, Warde Medical Laboratory recently reorganized its Virology testing menu in order to simplify orders for virus cultures and to provide additional rapid testing options. This report provides an explanation of these changes.
Simplified Culture Procedure
Virus culture is the gold standard for virus detection. To simplify this process, we have replaced 12 different source-driven (e.g., eye virus culture, genital virus culture, etc.) cultures with 3 new virus-based cultures, and a comprehensive virus culture. The pre-existing respiratory virus culture (RVC) and dermal virus culture (DVC) will continue to be offered. These changes were intended to simplify orders for virus isolation and bring our ordering practices in line with those of other reference laboratories. A symptom-based culture guide is provided to assist in the transition to the new test codes.
Comprehensive Virus Culture (CVC) — This procedure is used to order virus isolation from nearly every source. Turnaround times range from 1 to 22 days after specimen collection. CVC orders may include Cytomegalovirus (CMV), herpes simplex virus (HSV) and varicella-zoster virus cultures. Virus testing algorithms will vary depending upon the specimen source, time of year (some viruses are seasonal), age, and sex of the patient.
Cytomegalovirus Culture (CMVC) — Turnaround time is 5 days from date of collection. Urine, blood, and bronchoalveolar specimens should be kept refrigerated. Stool testing will not be performed.
Herpes Virus Culture (HVC) — Herpes simplex virus can be ordered from most sources except stool. Turnaround time is 2-8 days from date of collection. Herpes virus typing (HVT) may be ordered with the culture to decrease the turnaround time. The typing order will be cancelled if the culture is negative. Best results are obtained when vesicular lesions are unroofed and the base of the lesion is rubbed vigorously with a swab. Place the swab into viral transport medium and ship to the laboratory refrigerated.
Varicella-Zoster Virus Culture (VZVC) — Turnaround time is 6 days from time of collection. Best results are obtained when vesicular lesions are unroofed and the base of the lesion is rubbed vigorously with a swab. Place the swab into viral transport medium and ship to the laboratory refrigerated.
Dermal Virus Culture (DVC) — This precedure tests for the presence of herpes simplex virus (HSV) and varicella-zoster virus (VZV). This test is useful for vesicular lesions. Historical data indicates that approximately 30% of non-genital VZV cultures are positive for HSV. The best results are obtained when vesicular lesions are unroofed and the base of the lesion is rubbed vigorously with a swab. Place the swab into viral transport medium and ship to the laboratory refrigerated.
Respiratory Virus Culture (RVC) — Positive test results are usually available 3-5 days after the time of collection. Negative results are available in 11-15 days. Nasal washes and aspirates may be submitted. The best specimen is a nasopharyngeal (NP) swab plus a throat swab with both swab placed in the same viral transport tube. Ship the tube to the laboratory refrigerated.
Improved Third-Party Reimbursement
Proper coding for third-party reimbursement is an ongoing problem in the clinical laboratory. The new Virology procedures have been carefully scrutinized with regard to CPT-4 coding and to assure that the new billing codes conform to the 2001 coding changes. The new billing and CPT-4 codes more accurately represent the labor and cost inputs than the old codes.
Rapid Testing Options
Although virus culture is the gold standard, virus instability and long isolation times (days to weeks) can limit the clinical utility of some virus cultures. Several studies have shown that direct fluorescent antibody (DFA) methods are faster and in some cases, more sensitive than traditional cultures. In contrast with 2-10 day isolation times, DFA staining results are usually available in less than 24 hours.
DFA methods are very specific but DFA methodologies are not without problems. DFA reagents can only detect specific antigens. Therefore, DFA could miss specimens containing more than one virus unless multiple tests are requested. Exclusive use of DFA reagents could also miss new infectious agents that might appear in the community. For these reasons, Warde Medical Laboratory employs a combination of DFA and culture methods for routine virus detection.
The success of the DFA procedure depends upon the submission of specimens that contain virus-infected cells. Submission of acellular specimens can cause false-negative results because no positive cells will be observed after staining. Specimens with less than 1-2 cells/high power (400X) field will be reported as “Too Few Cells for Accurate Interpretation” rather than “Negative.”
Testing Algorithms — DFA testing is faster and less expensive than culture procedures. DFA procedures are also less sensitive than culture (except for RSV and VZV). Thus, a DFA-negative specimen could be culture-positive.
- For the fastest time to result, clients should order the appropriate DFA test (see symptom-based culture guide).
- For maximum sensitivity and when turnaround time is not an issue, clients should order virus cultures.
- For the fastest turnaround times and maximum sensitivity, clients should order DFA and culture procedures(Table 1). Culture orders will be canceled for specimens that are DFA-positive.
| Table 1: Suggested testing algorithms for HSV, VSV and respiratory virus testing. | |
| Desired Results | Suggested Test |
| Fastest time to result | DFA |
| Maximum sensitivity | Culture – RSV DFA if RSV is suspected. |
| Fastest turnaround time and maximum sensitivity | DFA and Culture – (The laboratory will cancel the culture if the DFA test is positive.) |
Respiratory Syncytial Virus DFA (RSV) — RSV is one of the major causes of acute respiratory illness in infants and children, causing up to 40% of pneumonia cases and 90% of bronchiolitis cases during the first months of life. Detection of RSV in cell culture is problematic because the virus is very thermolabile. (1) DFA methods are significantly more sensitive than culture, especially in outpatient settings.
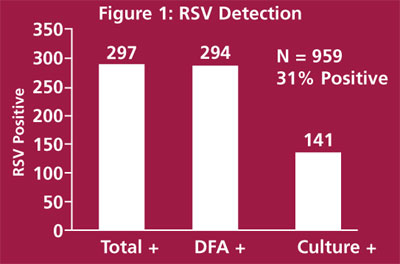
Figure 1 shows the results from 959 respiratory cultures sent to the laboratory for testing. DFA and culture procedures were performed on all specimens. A total of 297 specimens (31%) were RSV-positive by any method. DFA detected 294/297 (99%) of positive specimens while cell culture detected 141/297 (47%) of the positive specimens. These data are consistent with those published in the clinical virology literature.
Nasal washes and aspirates may be submitted for RSV DFA (RSV) testing. However, the best specimen is a nasopharyngeal (NP) swab plus a throat swab with both swabs placed in the same viral transport tube. Ship the tube to the laboratory refrigerated.
Respiratory Virus DFA Panel (RVD) — Respiratory virus DFA testing can significantly reduce respiratory virus detection times and can make a positive contribution to patient care. The sensitivity of this procedure depends upon which viruses are circulating in the community. Overall, the sensitivity is between 90% and 100% for the seven viruses detected by this procedure (influenza A, influenza B, adenovirus, RSV, parainfluenza type 1, type 2, and type 3.)
The sensitivity of RSV DFA testing is about 99% and the sensitivity of parainfluenza DFA testing is 93-99%. DFA testing is very reliable for these viruses. The sensitivity of influenza DFA is 93 -97% and our breakthrough rates are very low when these viruses predominate.
Adenovirus DFA testing is not as sensitive as the previously mentioned viruses. The sensitivity of this procedure is approximately 85%. DFA may be used for adenovirus testing but DFA-negative specimens should be cultured to maximize sensitivity.
Nasal washes and aspirates may be submitted for Respiratory Virus DFA Panel (RSD) testing. However, the best specimen is a nasopharyngeal (NP) swab plus a throat swab with both swabs placed in the same viral transport tube. Ship the tube to the laboratory refrigerated.
Varicella-zoster virus DFA (VZVD) — Varicella-zoster virus detection is a relatively low volume procedure in our laboratory because most cases of varicella (chickenpox) and herpes zoster (shingles) can be diagnosed by physical examination. For problematic cases, rapid DFA testing for VZV can significantly reduce the time-to-result and can serve as a guide for antiviral therapies. The data shown below indicate that the DFA procedure for VZV is at least as sensitive as culture.
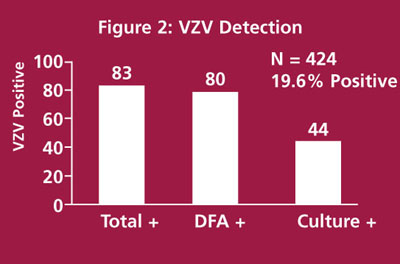
Figure 2 shows the results from 423 consecutive specimens submitted for VZV cultures. DFA and culture procedures were performed on each specimen. Eighty-three (19.6%) of these specimens were VZV-positive by any method. DFA detected 80/83 (96%) positive specimens while culture methods detected only 44/83 (53%) positive specimens. These data suggest that DFA procedures are sufficient for most dermal specimens.
Acellular (vesicle fluid) specimens are not recommended for DFA testing. Bronchoalveolar lavage, tissue specimens, and acellular specimens should be cultured. Ocular and spinal fluid specimens should be tested by PCR. Best results are obtained when vesicular lesions are unroofed and the base of the lesion is rubbed vigorously with a swab. Place the swab into viral transport medium and ship to the laboratory refrigerated.
Herpes Simplex Virus DFA (HSVD) — Detection of HSV by DFA is not as clear-cut as VZV detection. DFA methods are specific but the sensitivity depends upon how the specimen is collected. (2) With good specimen collection, DFA methods are at least as sensitive as cell culture isolation, particularly in an outpatient setting where specimens are transported for long distances.
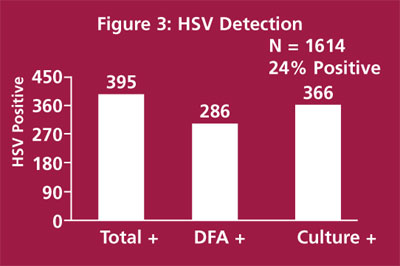
A herpes sensitivity study is shown in Figure 3. In this study we compared culture and DFA methods on 1614 consecutive specimens submitted for HSV culture. A total of 395 specimens (24%) were positive by any method. DFA detected 286/395 (72%) positive specimens and culture detected 366/395 (93%). The majority of discordant specimens (culture positive, DFA negative) had too few cells for adequate interpretation. These data suggest that DFA procedures may be used for dermal specimens when there are sufficient cells in the specimen for interpretation.
Acellular (vesicle fluid) specimens are not recommended for DFA testing. Best results are obtained when vesicular lesions are unroofed and the base of the lesion is rubbed vigorously with a swab. Place the swab into viral transport medium and ship to the laboratory refrigerated.
HSV PCR has proven to be an excellent method for detecting HSV encephalitis because the HSV isolation rate is only 5% for early in disease. PCR methods are also used to identify HSV in intra-ocular fluids.
HSV Typing from DFA — Clients should note that the mnemonic (HST) is different when HSV typing is done in conjunction with a DFA order rather than a culture order. Like the culture procedure, herpes typing may be ordered at the same time as the DFA test in order to reduce the time-to-result.
For More Information
Clients who want more information about viral testing, specimen collection, and/or ordering information for the new virology procedures should contact Dr. Dan Wiedbrauk, Mary Lu Barth, or the Virology Laboratory at Warde Medical Laboratory.
(734-665-8300 or 800-876-6522)
Literature Cited
- Hall CB. Respiratory syncytial virus. In: Mandell GL, Douglas RG Jr, Bennett JE, eds. Principles and Practice of Infectious Diseases, Third Edition. New York: Churchill Livingstone 1990;1265-1279.
- Green TA, Black CA, Johnson RE. Evaluation of bias in diagnostic-test sensitivity and specificity estimates computed by drescrepant analysis. J Clin Microbiol 1998;36:375-381.

