Vitamin D and Metabolites
Chemistry and Sources
There are two forms of vitamin D, vitamin 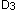 and vitamin
and vitamin 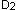 . Vitamin
. Vitamin 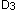 (cholecalciferol) occurs in some foods but is primarily produced in the skin on exposure to sunlight. Vitamin
(cholecalciferol) occurs in some foods but is primarily produced in the skin on exposure to sunlight. Vitamin 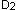 (ergocalciferol) does not occur naturally but is manufactured by the ultraviolet irridation of ergosterol, which occurs in molds, yeasts, and plants. Vitamin
(ergocalciferol) does not occur naturally but is manufactured by the ultraviolet irridation of ergosterol, which occurs in molds, yeasts, and plants. Vitamin 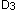 differs from vitamin
differs from vitamin 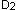 in that
in that 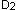 has a double bond between carbon 22 and carbon 23 and a methyl group on carbon 24 (Figure 1).
has a double bond between carbon 22 and carbon 23 and a methyl group on carbon 24 (Figure 1).
Only a few foods (cod liver oil, fatty fish, and egg yolks) contain substantial amounts of naturally occurring vitamin 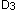 . Unless one has a diet rich in these foods, the amount of vitamin D obtained from food sources and skin synthesis often is insufficient. For this reason food is supplemented with vitamin D in most developed countries. In North America, food is supplemented with both vitamin
. Unless one has a diet rich in these foods, the amount of vitamin D obtained from food sources and skin synthesis often is insufficient. For this reason food is supplemented with vitamin D in most developed countries. In North America, food is supplemented with both vitamin 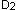 and vitamin
and vitamin 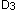 , with milk being the principal fortified dietary component.
, with milk being the principal fortified dietary component.
Transport and Metabolism
Once either vitamin 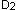 or
or 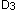 enters the circulation, whether ingested or made cutaneously, it is bound to vitamin D-binding protein and transported to the liver where it is hydroxylated on carbon position 25 to produce 25-hydroxyvitamin D (25(OH) vitamin D). 25(OH) vitamin D is released into the circulation once again before reaching the kidney. In the kidney, 25(OH) vitamin D undergoes a second hydroxylation at the 1
enters the circulation, whether ingested or made cutaneously, it is bound to vitamin D-binding protein and transported to the liver where it is hydroxylated on carbon position 25 to produce 25-hydroxyvitamin D (25(OH) vitamin D). 25(OH) vitamin D is released into the circulation once again before reaching the kidney. In the kidney, 25(OH) vitamin D undergoes a second hydroxylation at the 1 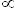 position to produce 1, 25-dihydroxyvitamin D (1, 25
position to produce 1, 25-dihydroxyvitamin D (1, 25 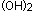 vitamin D) (Figure 2). (1)
vitamin D) (Figure 2). (1)
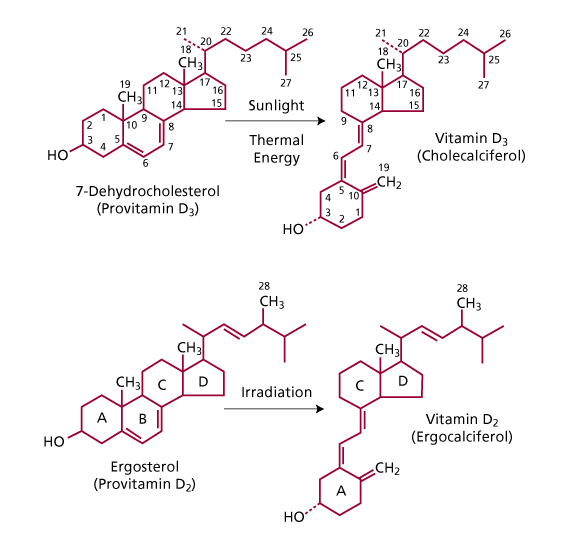
Figure 1: Structure of vitamin (cholecalciferol) and vitamin (ergocalciferol) and their precursors. 7-Cholecalciferol is produced in the skin from 7-dehydrocholesterol on exposure to sunlight. Ergocalciferol is produced commercially by irradiation of ergosterol. (Adapted from Endres, D.B. and Rude, R.K.: Mineral and Bone Metabolism. In: Tietz. Textbook of Clinical Chemistry and Molecular Diagnotics. 4th ed. Elsevier Saunders. St. Louis, Missouri. 2005: p. 1920. reproduced with permission of the publisher.)
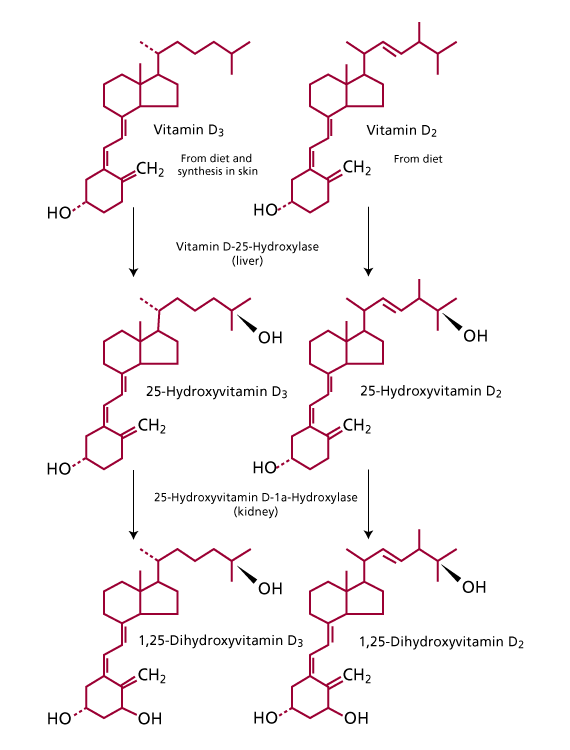
Figure 2: Metabolism of Vitamin D.Vitamin 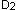 and Vitamin
and Vitamin 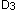 are enzymatically hydroxylated to 25-hydroxyvitamin D in the liver and 1,25 di-hydroxyvitamin D by the kidneys. 1,25 di-hydroxyvitamin
are enzymatically hydroxylated to 25-hydroxyvitamin D in the liver and 1,25 di-hydroxyvitamin D by the kidneys. 1,25 di-hydroxyvitamin 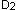 and 1,25 di-hydroxyvitamin
and 1,25 di-hydroxyvitamin 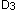 are the biologically active forms of vitamin D. (Adapted from Endres, D.B. and Rude, R.K.: Mineral and Bone Metabolism. In: Tietz. Textbook of Clinical Chemistry and Molecular Diagnotics. 4th ed. Elsevier Saunders. St. Louis, Missouri. 2005: p. 1921. reproduced with permission of the publisher.)
are the biologically active forms of vitamin D. (Adapted from Endres, D.B. and Rude, R.K.: Mineral and Bone Metabolism. In: Tietz. Textbook of Clinical Chemistry and Molecular Diagnotics. 4th ed. Elsevier Saunders. St. Louis, Missouri. 2005: p. 1921. reproduced with permission of the publisher.)
Biological Actions
25(OH) vitamin D is the major circulating form of vitamin D, but, at physiological concentrations, it is biologically inactive. Although biologically inactive, 25(OH) vitamin D has a longer half-life and is the preferred analyte to measure a patient’s vitamin D status. (2)
The active form of vitamin D is 1, 25 (OH) 2 vitamin D. (3) It is a hormone that responds to decreased serum calcium levels by stimulating the absorption of calcium and phosphate in the intestine and by enhancing parathyroid hormone-induced bone resorption. Elevations in serum calcium produce the opposite effect, that is, decreased intestinal calcium and phosphate absorption and decreased PTH-induced bone resorption.
Clinical Significance
A large number of disorders of bone and mineral metabolism have been associated with abnormal levels of vitamin D and its metabolites (Tables 1 and 2). (4) Although there are many disorders associated with abnormal plasma concentrations of 25 (OH) vitamin D and 1, 25 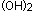 vitamin D, the most common disorder is vitamin D deficiency.
vitamin D, the most common disorder is vitamin D deficiency.
Table 1: Abnormal Circulating Levels of 25(OH) Vitamin D
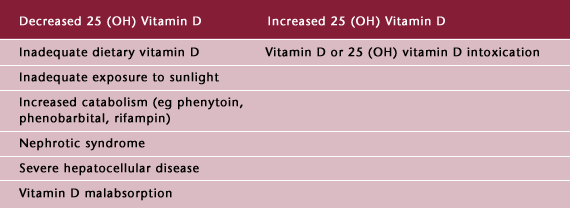
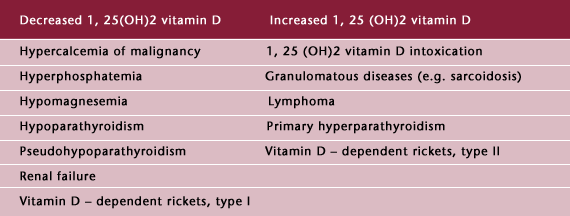
Table 2: Abnormal Circulating Levels of 1, 25 Vitamin D
Prolonged vitamin D deficiency leads to osteomalacia, a condition characterized by insufficient mineralization of the newly formed bone. Defective mineralization in childhood leads to rickets, and if untreated, produces severe deformities of bone. Osteomalacia in adults presents more subtly. Classic symptoms of osteomalacia in adults include bone pain, muscle weakness, with skeletal tenderness, and radiographic evidence of generalized thinning of cortical and trabecular bone (demineralization) with poorly healed stress fractures. Biochemically, patients have characteristic increases in PTH, low urinary calcium excretion, and increased indices of bone turnover including alkaline phosphatase and collagen crosslinks, and a deficiency of 25 (OH) vitamin D. (5,6)
Vitamin D deficiency is now recognized as common in elderly people, and the incidence may vary from 5-25% among independent elderly to 60-80% among institutionalized elderly depending on latitude, nutrition, sun exposure, supplementation, use of sunscreens, skin pigmentation, and clinical definition. (7) Opinions vary widely on the definition of vitamin D deficiency and adequate vitamin D status. (7,8,9,10,11,12,13)
A further discussion of the definition of vitamin D status is given below in the reference interval section.
Vitamin D toxicity, though less common than vitamin D deficiency, does occur. It can result from excessive ingestion of vitamin D, vitamin D analogues, or excessive use of topical calcipotriene (Dovonex) for psoriasis. Vitamin D intoxication can be recognized by hypercalcemia, hyperphosphatemia, and increased levels of 25 (OH) vitamin D.
An endogenous form of vitamin D intoxication, due to extrarenal conversion of 25 (OH) vitamin D to 1, 25 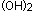 vitamin D, occurs in sarcoidosis (14), cat-scratch disease (15), and certain lymphomas (16). These patients have increased levels of 1, 25
vitamin D, occurs in sarcoidosis (14), cat-scratch disease (15), and certain lymphomas (16). These patients have increased levels of 1, 25 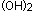 vitamin D. This endogenous form of vitamin D intoxication is, in many reports, the third most common cause of hypercalcemia, following primary hyperparathyroidism and malignancy.
vitamin D. This endogenous form of vitamin D intoxication is, in many reports, the third most common cause of hypercalcemia, following primary hyperparathyroidism and malignancy.
Reference Values
The reference range for 25 (OH) vitamin D is 10-52 ng/ml. This is based on the 95th percentile of apparently healthy adults in the United States. However, many clinicians have observed subclinical hypovitaminosis D in their patients in the low “normal” range. As a result, several studies have been conducted to determine adequate vitamin D status. In these studies, adequate vitamin D status was determined by comparing parathyroid hormone (PTH) levels before and after a period of vitamin D and calcium supplementation. Those individuals whose PTH levels did not significantly change after vitamin D supplementation were considered to have adequate vitamin D status; while individuals whose PTH levels were initially elevated and had their PTH levels decline after vitamin D supplementation were considered vitamin D insufficient. From these studies, the lower limit for adequate vitamin D status was estimated to be 20 or 30 ng/ml of 25 (OH) D. (7,8,13)
However, these studies, although highly suggestive, were based on physiological evidence, and did not prove a connection with morbidity. The publication in 2003 of a large British intervention study provided a crucial piece of needed evidence. With a placebo-controlled, randomized design, Trivedi at al (17) administered 100,000 IU of vitamin D every four months, for five years, to 2686 healthy British participants 65-85 years of age. Serum 25(OH) Vitamin D concentrations were measured and were 21 ng/ml for the placebo group and 30 ng/ml for the Vitamin D treated group. Among the treated group, the risk of all fractures was reduced by 22% and typical osteoporatic fractures were reduced by 33%.
The Vitamin D Dilemma
Based on the above, many clinicians have begun measuring 25(OH) Vitamin D levels in their patients and have initiated therapy when the 25(OH) Vitamin D levels are less than 30 ng/ml. The dilemma lies in the fact that, using this approach, nearly half of the world’s population would require treatment for hypovitaminosis D. For example, in a study by Gordon et al (18), at a lower limit of 20 ng/ml, 42% of healthy adolescents in Boston were vitamin D deficient or insufficient. Using a lower limit of 30 ng/ml, MacFarlane et al (19), found that 75% of healthy adults in Belgium would be similarly classified, and Rucker et al (20) found that 97% of healthy adults in Calgary, Alberta, would again, be classified as vitamin D deficient or insufficient.
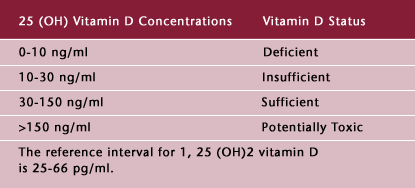
To date, there is no definitive classification of 25(OH) Vitamin D levels and Vitamin D status. Based on the available literature, we suggest the following classification (Table 3).
Table 3: Suggested Classification of Vitamin D Status
Treatment of Vitamin D Deficiency
Vitamin D deficiency is most commonly treated with calciferol (Drisdol®, “vitamin  “). As stated above, a dilemma exists on who and when to treat a patient for vitamin D deficiency or insufficiency. The interested reader is referred to the recommendations of the Australian Family Physicians (21) and to the paper by Wilkins and Birge (22), which provides specific recommendations on the prevention of osteoporotic fractures in the elderly.
“). As stated above, a dilemma exists on who and when to treat a patient for vitamin D deficiency or insufficiency. The interested reader is referred to the recommendations of the Australian Family Physicians (21) and to the paper by Wilkins and Birge (22), which provides specific recommendations on the prevention of osteoporotic fractures in the elderly.
Analytical Methodology
Of the more than 35 metabolites of vitamin 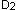 and vitamin
and vitamin 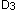 that have been identified only the measurement of 25 (OH) vitamin D and 1, 25
that have been identified only the measurement of 25 (OH) vitamin D and 1, 25 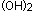 vitamin D has proven clinical value. For clinical purposes, the 25 (OH) vitamin D and 1, 25
vitamin D has proven clinical value. For clinical purposes, the 25 (OH) vitamin D and 1, 25 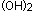 vitamin D assays should measure both 25 (OH) vitamin
vitamin D assays should measure both 25 (OH) vitamin 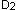 and 25 (OH) vitamin
and 25 (OH) vitamin 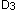 or 1, 25
or 1, 25 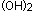 vitamin
vitamin 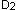 and 1, 25
and 1, 25 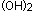 vitamin
vitamin 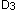 . Separate measurements of the vitamin
. Separate measurements of the vitamin 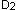 and vitamin
and vitamin 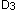 forms does not provide useful information or an indication of dietary and endogenous vitamin D because food is supplemented with both vitamin
forms does not provide useful information or an indication of dietary and endogenous vitamin D because food is supplemented with both vitamin 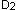 and vitamin
and vitamin 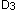 .(3,23) Because both vitamin
.(3,23) Because both vitamin 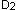 and vitamin
and vitamin 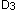 are metabolized to produce dihydroxylated metabolites of nearly equal biological activity, vitamin D assays should measure metabolites of both vitamin
are metabolized to produce dihydroxylated metabolites of nearly equal biological activity, vitamin D assays should measure metabolites of both vitamin 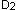 and vitamin
and vitamin 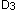 , with equi-molar cross-reactivity.(24,25) Warde Medical Laboratory utilizes a chemiluminesent assay for 25 (OH) vitamin D and a radioimmunoassay for 1, 25
, with equi-molar cross-reactivity.(24,25) Warde Medical Laboratory utilizes a chemiluminesent assay for 25 (OH) vitamin D and a radioimmunoassay for 1, 25 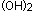 , vitamin D, both with equi-molar cross-reactivity.
, vitamin D, both with equi-molar cross-reactivity.
References
- Endres, D.B. and Rude, R.K.: Mineral and Bone Metabolism. In: Tietz Textbook of Clinical Chemistry, 3rd ed., C.A. Brutis, E. R. Ashwood, Eds. Philadelphia, E.B. Saunders, 1999, pp. 1417-1418.
- Preece, M.A., S. Tomlinson, C.A. Ribot, J. Pietrek, H.T. Korn, D.M. Davies, J.A. Ford, M.G. Dunnigan, and J.L.H. O'Riordan. 1975. Studies of Vitamin D deficiency in man. Quarterly Journal of Medicine 44:757.
- Holick MF. Vitamin D: Photobiology, Metabolism, Mechanism of Action, and Clinical Applications. In: Favus MJ, editor. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism, 4th Edition. Lippincott Williams & Wilkins, Philadelphia, PA., 1999: 92-98.
- Adapted from Endres, D.B. and Rude, R.K.: Mineral and Bone Metabolism. In: Tietz Textbook of Clinical Chemistry, 3rd ed., C.A. Brutis, E. R. Ashwood, Eds. Philadelphia, E.B. Saunders, 1999, pp. 1422-1423.
- Rosen CJ. Vitamin D and Bone Health in Adults and the Elderly. In: Holick MF, editor. Vitamin D: Physiology, Molecular Biology, and Clinical Applications. Humana Press, Totowa, NJ. 1999:287-305.
- Luckey MM. Evaluation of Postmenopausal Osteoporosis. In: Favus MJ, editor. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism, 4th Edition. Lippincott Williams & Wilkins, Philadelphia, PA., 1999: 273-277.
- Malabanan A, Veronikis IE, Holick MF. Redefining vitamin D deficiency. Lancet 1998; 351:805-806.
- Chapuy M-C, Preziosi P, Maamer M. et al. Prevalance of Vitamin D insuffiency in an adult normal population. Osteoporosis Int 1997; 7:439-443.
- Dawson-Hughes B. Calcium, vitamin D and vitamin D metabolites. In: Papapoulos SE, Lips P, Pols HAP, Johnston CC, Delmas PD, eds. Osteoporosis 1996. Proceedings of the 1996 World Congress on Osteoporosis. Excerpt Med Int Congr Ser 118. Amsterdam: Elsevier; 299-303.
- Lips P, Wiersinga A, Ginkel van FC, et al. The effects of vitamin D supplementation on vitamin D status and parathyroid function in elderly subjects. J Clin Endocrinol Metab. 1988; 67:644-650.
- Thomas MK, Lloyd-Jones DM, Thadhani Rl, et al. Hypovitaminosis D in medical patients. N Engl J Med. 1998; 338:777-783.
- Vieth R. Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. Am J Clin Nutr. 1999; 69:842-856.
- Lips P, Duong T, Oleksik A, Black D, Cummings S, Cox D, Nickelsen T. A global study of vitamin D status and parathyroid function in postmenopausal women with osteoporosis: baseline data from the multiple outcomes of Raloxifene evaluation clinical trial. J Clin Endocrinol Metab 2001; 86:1212-1221.
- Young C, Burrows R, Katz J, et al, "Hypercalacaemia in Sarcoidosis," Lancet, 1999, 353(9150):374.
- Bosch X, "Hypercalcemia Due to Endogenous Overproduction of Active Vitamin D in Identical Twins With Cat-Scratch Disease," JAMA, 1998, 279(7):532-4.
- Seymour JF, Gagel RF, Hagemeister FB, et al, "Calcitriol Production in Hypercalcemic and Normocalcemic Patients With non-Hodgkin Lymphoma," Ann Intern Med, 1994, 121(9):633-40.
- Trivedi DP, Doll R, Khaw KT. Effect of four monthly oral vitamin
(cholecalciferol) supplementation on fractures and mortality in men and women living in the community; randomized double blind controlled trial. BMJ 2003;326:469-72.
- Gordon CM, DePeter KC, Feldman HA, Grace E, Emans SJ. Prevalence of vitamin D deficiency among healthy adolescents. Archives of Pediatrics and Adolescent Medicine 2004;158,531-7.
- MacFarlane GD et al. Hypovitaminosis D in a normal, apparently healthy, urban European population. Journal of Steroid Biochemistry and Molecular Biology 2004;89-90;621-2.
- Rucker D, Allan JA, Fick GH, Hanley DA. Vitamin D insuffiency in a population of healthy western Canadians. CMAJ 2002:166,1517-24.
- Nowson CA, Diamond TH et al. Vitamin D in Australia, Issues and Recommendations. Australian Family Physician Vol 33, No. 3, March 2004.
- Wilkins CH, Birge SJ. Prevention of osteoporotic fractures in the elderly. The Amercian Journal of Medicine (2005) 118,1190-1195.
- Holick, M.F.: Vitamin D: Photobiology, metabolism and clinical applications. In: Endocrinology. 3rd ed. Vol. 2. L.J. DeGroot, Ed. Philadelphia, W.B. Saunders, 1995, pp. 990-1014.
- Hollis, B.W. and W.B. Pittard III. 1984. Relative concentrations of 25- hydroxyvitamin
/
and 1,25-dihydroxyvitamin
/
in maternal plasma at delivery. Nutrition Research 4:27
- Hollis, B.W. and W.B. and W.B. Pittard III. 1984. Evaluation of the total fetomaternal Vitamin D relationships at term: Evidence for racial differences. Journal of Clinical Endocrinology and Metabolism 59:652.

